ISO 10993 Skin irritation Test
Skin contact is the most common route of exposure to medical devices. If medical device materials contain primary irritants, they may cause inflammatory lesions of the skin, which may manifest themselves as a direct damaging reaction characterized by inflammation, or as symptoms of severe irritation, skin blistering, and/or necrosis. Therefore, in order to evaluate whether a product or product extract has potential skin irritation, skin irritation testing is required. The skin irritation test belongs to the "three basic biocompatibility tests" and its scope of application is very wide.

Choice of Skin Irritation Test Methods
Skin irritation test is divided into two contact methods, namely direct contact method and extract test.
The two testing methods will also have slightly different operations:
▷ The direct contact method is to put the test material in direct contact with the contact site, and then fix it or fix it on the contact site with a dressing;
▷ The extraction solution test is different. It requires adding drops of the extraction solution prepared in advance to the dressing, applying it to the contact area and then fixing it.
So, how to choose the above two contact methods for different samples? The table below will be displayed visually for everyone to choose from:
Sample type | direct contact method | Extract test |
solid | Be applicable | Be applicable |
liquid | Be applicable | not applicable |
powder | Be applicable | not applicable |
Skin irritation test method
The test sample or the gauze containing the test sample extract was applied to the test site on the back of the three experimental animals (as shown in Figure 1). The corresponding extraction medium without the test sample was used as a blank control and was applied to the control site. Remove the application after 4 hours; observe the application site immediately, 1 hour, 24 hours, 48 hours and 72 hours after removal of the application and record the observation results.
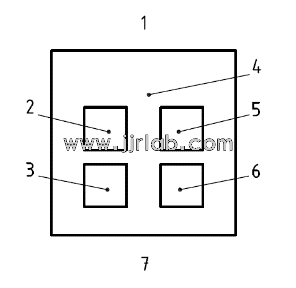
Figure 1 Application location
1-head end; 2-test site (1); 3-control site (1); 4-hairless back area; 5-control site (2); 6-test site (2); 7-tail end
evaluate
Observe skin reactions under natural light or full-spectrum lighting. And according to the scoring system given in Table 1, describe and score the skin erythema and edema reaction of each contact part within each specified time, and record the results.
Table 1 Skin reaction scoring system
Reaction (erythema and eschar formation) | stimulus score |
No erythema | 0 |
Very slight erythema (barely visible) | 1 |
Clear erythema | 2 |
Moderate erythema | 3 |
Severe erythema (purple red) to eschar formation | 4 |
Reaction (edema formation) |
_ |
No edema | 0 |
Very slight edema (barely visible) | 1 |
Clear edema (swelling that does not extend beyond the edge of the area) | 2 |
Moderate edema (swelling about 1 mm) | 3 |
Severe edema (swelling greater than 1 mm and extending beyond the contact area) | 4 |
Stimulate the highest score | 8 |
After recording the results, finally judge the test results based on the values shown in Table 2 and draw the test conclusion.
Table 2 Types of stimulation index
reaction type | average score |
extremely slight | 0~0.4 |
Mild | 0.5~1.9 |
Moderate | 2~4.9 |
Severe | 5~8.0 |
cycle positive control
Skin irritation test results may vary due to changes in many test-related factors, such as host, test dose, patch size, degree of occlusion, contact time, medium, reading time, and reading quality. Therefore, it is important to include known positive and negative control materials in skin irritation tests so that test and control materials can be compared to produce appropriate results. Sodium dodecyl sulfate (SDS) with a purity of ≥99% is the preferred positive control material. SDS is the most widely used control irritant in clinical research and has no other adverse effects.
Safety evaluation studies (in vivo and in vitro) of medical devices are used to determine the safety or biocompatibility of devices in biological environments. Biological evaluation includes screening tests of new materials from the initial R&D stage, regular evaluation tests and non-clinical tests or pre-market safety assessments to ensure that the device meets the standard requirements for entry into the target market. The Haihe Marking Team provides comprehensive service solutions to domestic and foreign medical device companies. According to the target market selected by the company, it develops a complete test plan for products or raw materials, including the selection of test items, the establishment of plans, the implementation of tests, and support in the subsequent review process. It provides testing services for customers at different stages such as design and development, process confirmation, and preclinical research, and provides customers with product improvement solutions.