
Which products need ISO 18562 testing?
The ISO 18562 series standards apply to all medical devices, components, or accessories with gas pathways that have indirect patient contact. Common medical device products include ventilators, respiratory systems, anesthesia workstations (including gas mixers), oxygen concentrators, nebulizers, medical humidifiers, oxygen conserving devices, low-pressure hoses, heat and moisture exchangers, respiratory gas monitors, respiratory monitors, respiratory masks, ventilators, breathing tubes, respiratory system filters, and Y-connectors, as well as any similar medical device accessories. Additionally, the inner chambers of pediatric incubators, mattress covers, and the interior surfaces of oxygen masks are considered part of the gas pathway.
These gas pathways are intended to provide respiratory care or substance supply to patients through the respiratory tract in various environments.
The ISO 18562 series standards do not cover surfaces of devices that come into direct contact with patients; their biological evaluation should follow the ISO 10993 series standards. Furthermore, biological hazards caused by mechanical failures and gas source contamination in gas supplies are not covered by this standard.
JJR Laboratory in China is an ISO 17025 accredited institution offering iso 18562 testing services. Feel free to contact us for a quotation.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
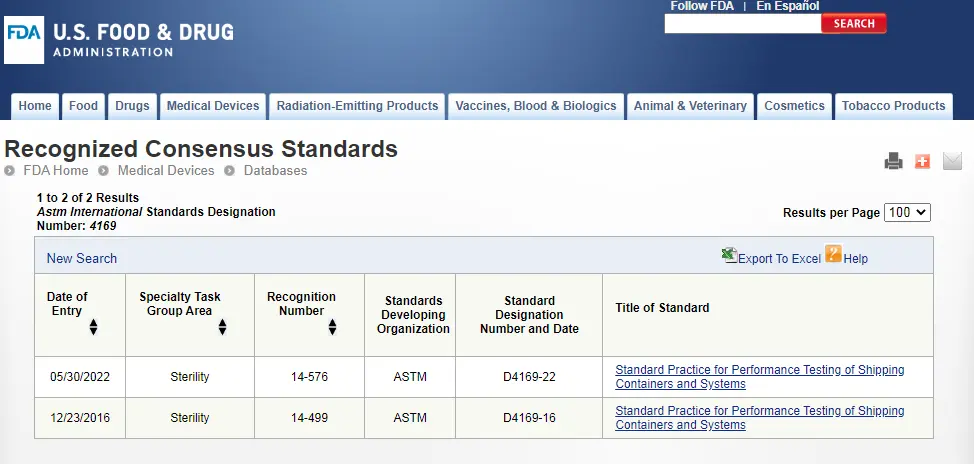 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
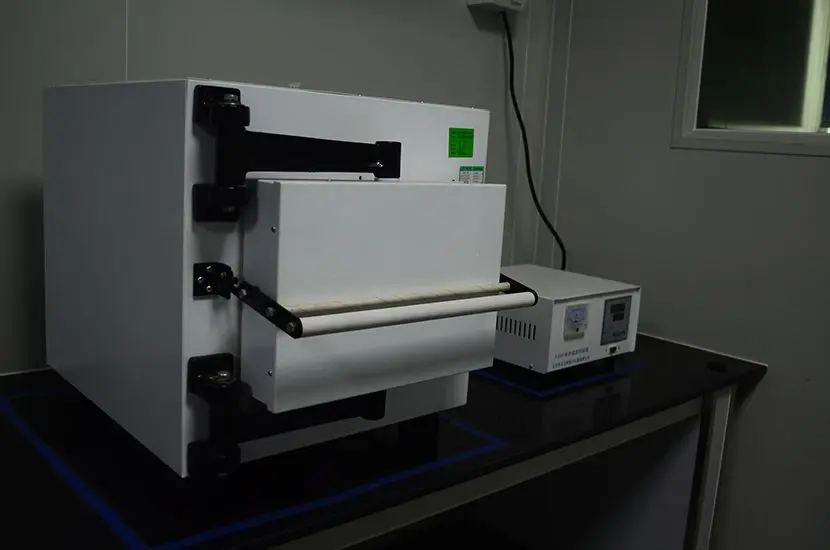 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




