
What is Japan's MIC certification?
Introduction to Japan's Mic certification
For many clients who need export certification, MIC is a commonly used certification. This certification is mandatory for wireless products entering the Japanese market. The MIC in MIC certification refers to the Ministry of Internal Affairs and Communications (MIC) of Japan. It oversees Japan's Radio Law and the Telecommunications Business Law. The Radio Law, previously known as telec certification, is also referred to as mic certification from a professional perspective. In short, TELEC certification is equivalent to MIC certification.

Scope of MIC certification applicable products
Wireless RF products include: Bluetooth products, ZigBee products, telemetry devices, WiFi products, wireless microphones, pagers, LTE•RFID (2.4GHz, 920MHz) products, UWB radio systems, GSM products, etc.
MIC certification process
1. Fill out the application form, prepare documents, and samples.
2. Testing institution reviews the documents and conducts preliminary testing of samples.
3. Testing institution formally submits the application to MIC accredited by the Ministry of Internal Affairs and Communications.
4. Review of documents by the institution.
5. Sample testing, providing test reports (can be tested by authorized laboratories).
6. Issuance of certificate by Japan's MIC after documents and test reports are approved.

Materials required:
1. Technical specification sheet
2. Declaration of quality management system
3. Quality control confirmation letter, method, or manufacturer's ISO 9001 certificate
4. Construction guarantee confirmation letter
5. Rated power declaration
6. Antenna report
7. Test report (including test results, test setup photos, and inspections conducted)
8. Diagrams, schematics, material lists, part placements, IC datasheets, internal and external photos, product descriptions, user manuals, operation/technical specifications, accessory descriptions
9. Label information (label location, label content)
10. If you are acting as an agent or authorized representative of the manufacturer, a letter of proxy/authorization is also required.
More:Japan JATE certification | Japan Telec Certification | Japan's METI Registration | Japanese PSE Certification | Japan's VCCI certification
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
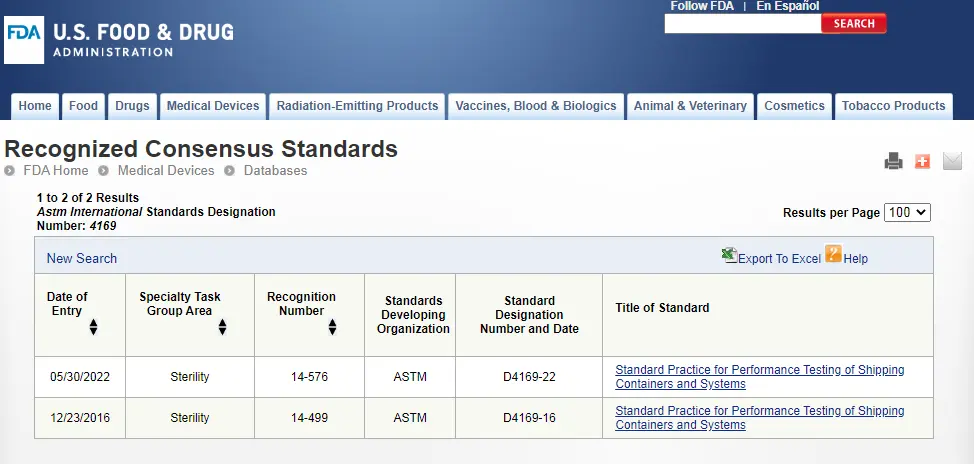 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
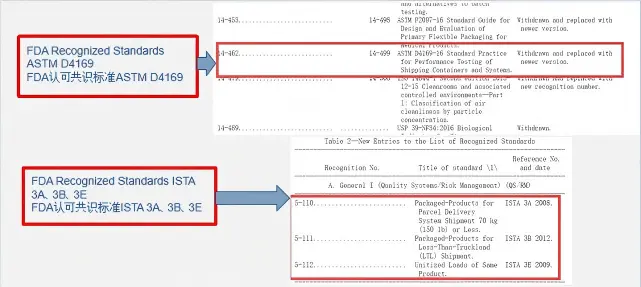
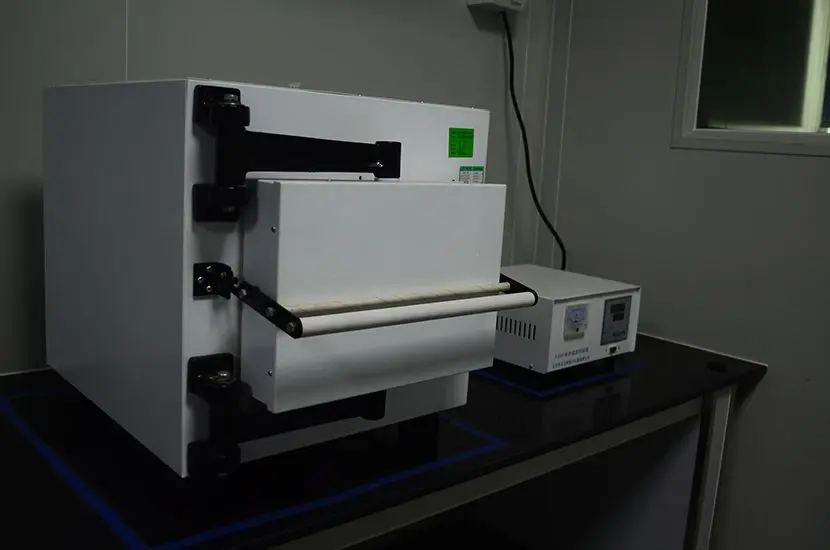 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




