
What is Biocompatibility Testing?
What is biocompatibility Test?
Biocompatibility refers to the property of life tissues reacting to inert materials, generally referring to the compatibility between materials and hosts. After implantation into the human body, biomaterials exert effects on specific biological tissue environments, while biological tissues also exert effects on biomaterials. This interaction continues until equilibrium is reached or the implant is removed.

Biocompatibility can be divided into biological reactions and material reactions. Biological reactions include blood reactions, immune reactions, and tissue reactions; material reactions mainly manifest as changes in material physical and chemical properties.
Biocompatibility is mainly determined by the nature and usage of the material. Properties of the material and product itself, including shape, size, and surface roughness, as well as toxic low-molecular-weight substances remaining from material polymerization or preparation processes, material processing contamination, and degradation products in the body, are all related to its biocompatibility. Short-term contact between materials and organisms can induce toxicity, irritation, teratogenicity, and local inflammation in cells and the body; long-term contact may have mutagenic, teratogenic, and carcinogenic effects; contact with blood can cause abnormal coagulation function and hemolysis. Therefore, when considering the use of materials in the biomedical field, their biocompatibility is an important indicator that needs to be considered and evaluated.

Which products need biocompatibility testing?
1. Active products related
Multiparameter monitors, ultrasound diagnostic equipment, infusion pumps and controllers, thermometers, non-invasive blood pressure monitoring equipment, pulse oximeters, high-frequency electrosurgical equipment, nerve and muscle stimulators, endoscopes, electroencephalographs, surgical, cosmetic, diagnostic, and therapeutic laser equipment, electrocardiograph equipment, clinical chemistry analyzers, immunochemistry analyzers, fully automatic blood analyzers, microbial analyzers, fully automatic protein analyzers, biochemical analyzers, blood cell analyzers, blood gas analyzers, chemiluminescence immunoassay analyzers, urine sediment analyzers, coagulation analyzers, fully automatic blood rheometers, fully automatic internal bacteria culture analyzers, microbial identification and bacterial susceptibility analyzers, nucleic acid purification instruments, blood and tissue culture instruments, cryostats, biological tissue dehydrators, tissue embedding machines, centrifuges, mixers, dyeing machines, high-temperature pulsating vacuum sterilizers, high-temperature steam sterilizers, infrared electric sterilizers, high-temperature disinfection cleaning machines, biological safety cabinets, etc.
2. Passive products related
1) Surface contact devices:
Electrodes, external prostheses, fixation straps, compression bandages, and various types of monitors;
Contact lenses, urinary catheters, intravascular or digestive tract instruments (gastric tubes, colonoscopes, gastroscopes), endotracheal tubes, bronchoscopes, etc.;
Dressings or nursing instruments and occlusive dressings for ulcers, burns, and granulation tissue, etc.
2) External access devices
Blood transfusion, infusion sets, extension sets, transfer sets, etc.
Laparoscopes, arthroscopes, drainage systems, dental filling materials, skin nails, etc.
Intravascular catheters, temporary pacing electrodes, dialyzers, dialysis tubing and accessories, vascular adsorbents, immunoadsorbents, etc.
3) Implantable devices
Orthopedic nails, artificial joints, bone substitutes, bone cement and intramedullary instruments, pacemakers, implantable drug delivery devices, nerve-muscle sensors and stimulators, artificial tendons, breast implants, artificial larynxes, subperiosteal implants, ligating clips, etc.
Pacemaker electrodes, artificial arteriovenous fistula tubes, heart valves, artificial blood vessels, intravascular drug delivery catheters, and ventricular assist devices.
What are the testing items for biocompatibility?
l In vitro cytotoxicity test
l Mouse pyrogenicity Salmonella mutagenicity test
l Sensitization test
l Gene mutation test
l Skin irritation test
l Thrombosis test
l Intradermal irritation test
l Coagulation test
l Acute systemic toxicity test
l Subacute systemic toxicity test
l Platelet adhesion test
l Complement activation test
l Subchronic systemic toxicity test
l Chronic systemic toxicity test
l Muscle implantation test
l Hemolysis test Heat source test
l Chromosomal aberration test
l Bone implantation test
l Material characteristic analysis
l Subcutaneous implantation test
Biocompatibility Testing Standards
1. Cytotoxicity Testing
• Cytotoxicity Test (MTT Method) (ISO 10993-5)
• Cytotoxicity Test (Agar Overlay Method) (ISO 10993-5 / USP 87)
• Cytotoxicity Test (Membrane Filtration Method) (ISO 10993-5)
• Cytotoxicity Test (Direct Contact Method) (ISO 10993-5 / USP 87)
• Cytotoxicity Test (Elution Method) (USP 87)
2. Skin Irritation and Sensitization Testing
• Sensitization Test (Maximal Dose Method / Patch Method) (iso 10993-10)
• Skin Irritation Test (ISO 10993-10)
• Intradermal Irritation Test (ISO 10993-10 / USP 88)
• Oral Irritation Test (Requires Histopathological Examination) (ISO 10993-10)
• Vaginal Irritation Test (Requires Histopathological Examination) (ISO 10993-10)
• Penile Irritation Test (Requires Histopathological Examination) (ISO 10993-10)
• Rectal Irritation Test (Requires Histopathological Examination) (ISO 10993-10)
• Eye Irritation Test (ISO 10993-10)
3. Systemic Toxicity Testing
• Acute Systemic Toxicity Test (ISO 10993-11 / USP 88)
• Subacute Systemic Toxicity Test (ISO 10993-11)
• Sub/Chronic Systemic Toxicity Test (ISO 10993-11)
• Pyrogen Test (ISO 10993-11)
4. Implantation After Local Reaction Testing
• Subcutaneous Implantation Test (ISO 10993-6)
• Muscle Implantation Test (ISO 10993-6)
• Bone Implantation Test (ISO 10993-6)
5. Blood Compatibility Testing
• Hemolysis Test (ISO 10993-4)
• Hemolysis Test (ASTM F756)
• Coagulation Test (ISO 10993-4)
• Platelet Count Test
• Complement Test (ISO 10993-4)
• Thrombosis Test (In Vivo, In Vitro) (ISO 10993-4)
6. Genotoxicity / Genetic Toxicity Testing (ISO 10993-3)
• Bacterial Reverse Mutation Test
• Mouse Lymphoma Test
• Chromosomal Aberration Test
• Micronucleus Test (Mouse)
How much does biocompatibility testing cost?
In Chinese laboratories, the cost of three biocompatibility tests conducted according to ISO 10993 standards is $3500 USD; of course, we need your test samples; (Email: chen18814186731@gmail.com)
Question and Answer Interpretation:
How to Choose Medical Device Biocompatibility Testing Items?
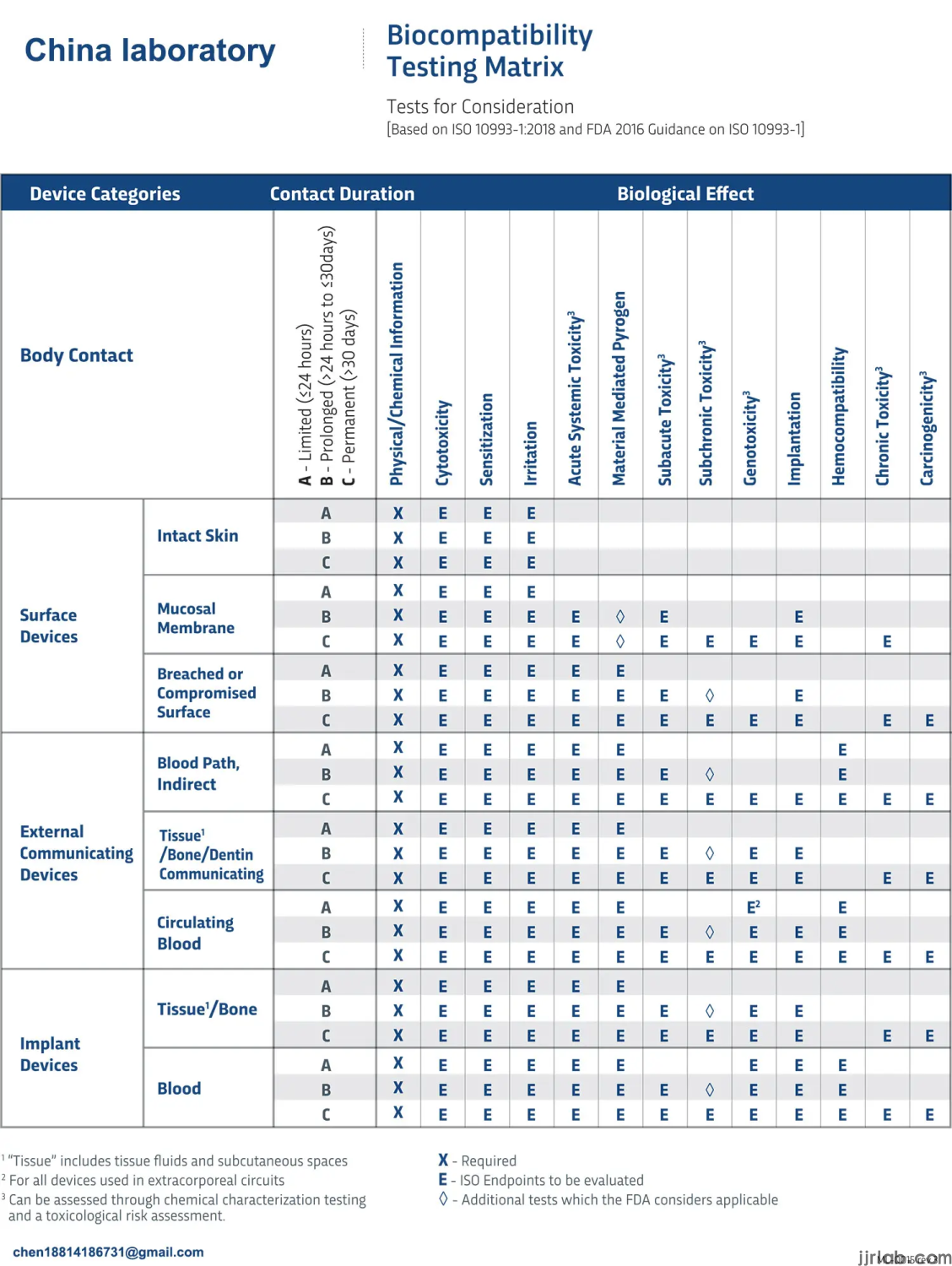
If everyone determines the category of the device according to the above two classification principles, they can then "find positioning" for the device based on Appendix A, Table 1 of standard ISO 10993-1:2018. This table clearly outlines the relationship between device categories and testing items, with the first column being the device category, the second column being the contact situation, the third column being the contact time, and the fourth column being the testing items.
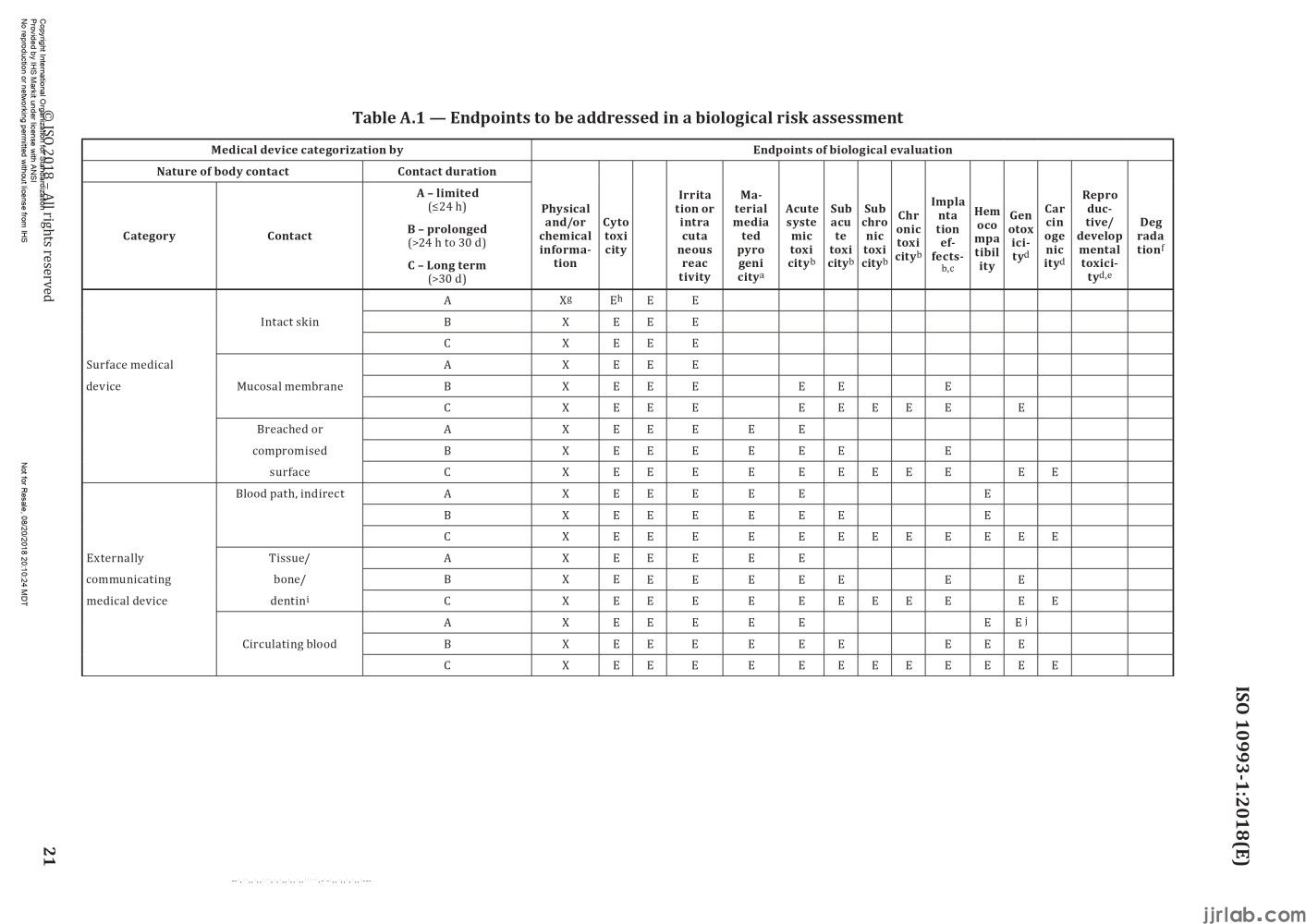
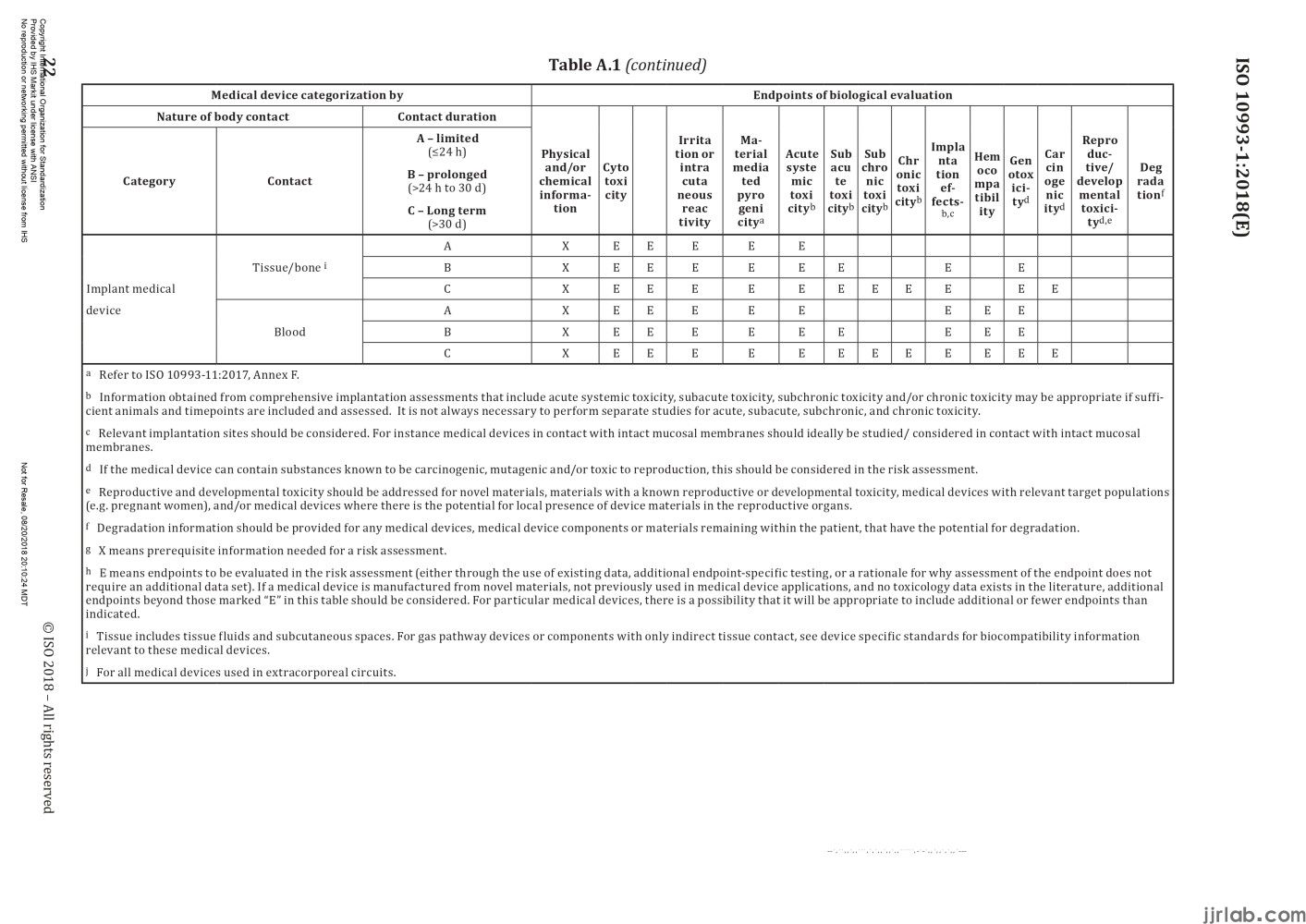
Taking a sphygmomanometer and a heart valve as examples, let's see which biocompatibility tests need to be conducted. The sphygmomanometer belongs to devices that come into contact with the body surface, the contact area is intact skin, and the contact time is less than 24 hours, corresponding to the first row of the table. Therefore, the tests to be conducted include in vitro cytotoxicity testing, irritation and sensitization testing, commonly referred to as the "three biocompatibility tests.
The heart valve belongs to implantable devices, the contact area is the circulating blood, and the contact time is more than 30 days, corresponding to the last row of the table. The tests to be conducted include, in addition to the "basic three tests," material-mediated pyrogenicity, acute toxicity, subacute toxicity, chronic toxicity, subchronic toxicity, local implantation effects, blood compatibility, genotoxicity, and carcinogenicity. Therefore, for high-risk products like heart valves, almost all testing items need to be covered. Through the above two examples, it is not difficult to find suitable testing items by using Table A.1 if the device category is clear.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




