
Ventilator Certification Testing Laboratory
Home Ventilator Therapy
Home ventilator therapy typically refers to non-invasive positive pressure ventilation, which is often used during the patient's recovery phase. The principle involves connecting the ventilator, which has pre-set parameters, to the patient's mouth and nose via a breathing circuit and mask. The airflow generated by the ventilator provides positive pressure to support the patient's airway. Additionally, the ventilator simulates and synchronizes with the patient's normal breathing frequency, assisting in the restoration of normal breathing to treat and improve oxygenation issues caused by ventilation disorders.

Types of Home Ventilators
Home ventilators are categorized into three types:
1. Life-supporting Ventilators: Provide or increase lung ventilation for patients who rely on ventilators.
2. Sleep Apnea Treatment Devices: Suitable for conditions such as snoring and obstructive sleep apnea syndrome.
3. Non-life-supporting Ventilators: In addition to treating sleep apnea syndrome, these are also suitable for treating mild to moderate respiratory disorders such as pulmonary heart disease, respiratory failure, chronic obstructive pulmonary disease (COPD), and neuromuscular weakness.
Suitable Users for Home Ventilators
1. Sleep Apnea Syndrome: Suitable for individuals with conditions like snoring and obstructive sleep apnea syndrome.
2. Chronic Obstructive Pulmonary Disease (COPD): COPD is a common chronic respiratory disease where patients experience narrowed airways and breathing difficulties. Home ventilators can help improve ventilation and reduce symptoms of breathlessness.
3. Patients with Neuromuscular Diseases: Conditions such as motor neuron disease and myasthenia gravis cause breathing difficulties due to neuromuscular abnormalities. Home ventilators can assist these patients in breathing, improving their quality of life.
4. Chronic Cardiopulmonary Disease Patients During Recovery: For some chronic cardiopulmonary disease patients, home ventilators can aid in rehabilitation training at home, enhancing lung function and exercise tolerance.
Usage Considerations for Home Ventilators
- Intolerance: Initially, patients may feel discomfort using a ventilator. Family members should provide guidance, adjust the mask, modify parameters, and offer psychological support. Once synchronization between the patient and the machine is achieved, discomfort will lessen.
- Oropharyngeal Dryness: Using a heated humidifier and drinking adequate water can alleviate dryness.
- Mouth Leakage: If using a nasal mask, try to keep the mouth closed to prevent leakage, which reduces the effectiveness. If necessary, use a full face mask or a chin strap and adjust the mask's tightness.
- Sputum Discharge Issues: Family members should help patients turn over and pat their backs regularly, encourage fluid intake, and guide effective coughing to expel sputum. Nebulized inhalation or suctioning may be required in some cases.
- Gastrointestinal Distension: Using a full face mask or opening the mouth during treatment can cause swallowing of air, leading to distension. If the nasal airway is unobstructed, use a nasal mask and nasal breathing. Adjust ventilator pressure if necessary and consider medications to enhance gastric motility under professional guidance.
Home ventilators are crucial medical devices for specific groups of people. Understanding their principles, suitable users, and usage considerations can help us better utilize home ventilators and safeguard our health.
JJRLAB Testing and Certification Solutions
EMC:
- yy 9706.102-2021: Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility requirements and tests
Safety Regulations:
- GB 9706.1-2020: Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
- YY 9706.111-2021: Medical electrical equipment - Part 1-11: General requirements for basic safety and essential performance - Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment
- YY/T 9706.106-2021: Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability
- YY 9706.108-2021: Medical electrical equipment - Part 1-8: General requirements for basic safety and essential performance - Collateral standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems
- GB/T 14710-2009: Environmental requirements and test methods for medical electrical equipment
Product Performance Standards:
- YY 9706.270-2021: Medical electrical equipment - Part 2-70: Particular requirements for the basic safety and essential performance of sleep apnea treatment devices
- YY 9706.272-2021: Medical electrical equipment - Part 2-72: Particular requirements for the basic safety and essential performance of home ventilators for ventilator-dependent patients
- YY 9706.274-2022: Medical electrical equipment - Part 2-74: Particular requirements for the basic safety and essential performance of respiratory humidification devices
- GB 9706.290-2022: Medical electrical equipment - Part 2-90: Particular requirements for the basic safety and essential performance of high flow respiratory therapy equipment
Performance Requirements for Ventilator Accessories:
- YY/T 0671-2021: Sleep apnea treatment masks and application accessories
- YY/T 0461-2003: Breathing tubes for anesthesia and ventilators
- YY/T 1040.1-2015: Conical connectors for anesthesia and respiratory equipment - Part 1: Cones and sockets
- YY/T 1040.2-2008: Conical connectors for anesthesia and respiratory equipment - Part 2: Screw-threaded weight-bearing connectors
Biocompatibility:
- GB/T 16886.1-2022: Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process
- GB/T 16886.5-2017: Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity
- GB/T 16886.10-2017: Biological evaluation of medical devices - Part 10: Tests for irritation and skin sensitization
- GB/T 16886.11-2021: Biological evaluation of medical devices - Part 11: Tests for systemic toxicity
- YY/T 1778.1-2021: Biological evaluation of respiratory gas pathways in healthcare applications - Part 1: Evaluation and testing within a risk management process
Software:
- GB/T 25000.51: Systems and software engineering - Systems and software quality requirements and evaluation (SQuaRE) - Part 51: Quality requirements and test details for ready-to-use software products (RUSP)
- GB/T 25000.10: Systems and software engineering - Systems and software quality requirements and evaluation (SQuaRE) - Part 10: System and software quality models
Shelf Life & Packaging and Transportation:
- YY 0681 Series: Testing of packaging for sterile medical devices
- GB/T 4857: Packaging and transportation testing
Sterilization Validation:
- GB 18280.2: Radiation sterilization dose setting
- GB/T 16886-7: Ethylene oxide sterilization residuals
- WS 310.1 / WS 310.2 / WS 310.3 / WS/T 367 / GB 18278.1 / YY/T 0734 / YY/T 1495: Cleaning, disinfection, and sterilization verification
International Standards
EMC:
- IEC 60601-1-2:2014+A1:2020: Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility requirements and tests
Safety Regulations:
- IEC 60601-1:2005/COR3:2022: Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
- IEC 60601-1-11:2015, AMD1:2020: Medical electrical equipment - Part 1-11: General requirements for basic safety and essential performance - Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment
- IEC 60601-1-6:2010+A1:2013+A2:2020: Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability
- IEC 60601-1-8:2006+A2:2020: Medical electrical equipment - Part 1-8: General requirements for basic safety and essential performance - Collateral standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems
Product Performance Standards:
- ISO 80601-2-70:2020: Medical electrical equipment - Part 2-70: Particular requirements for the basic safety and essential performance of sleep apnea treatment devices
- ISO 80601-2-72:2023: Medical electrical equipment - Part 2-72: Particular requirements for the basic safety and essential performance of home ventilators for ventilator-dependent patients
- ISO 80601-2-74:2021: Medical electrical equipment - Part 2-74: Particular requirements for the basic safety and essential performance of respiratory humidification devices
- ISO 80601-2-90:2021: Medical electrical equipment - Part 2-90: Particular requirements for the basic safety and essential performance of high flow respiratory therapy equipment
Biocompatibility:
- ISO 10993-1:2018: Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process
- ISO 10993-5:2009: Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity
- iso 10993-10:2021: Biological evaluation of medical devices - Part 10: Tests for skin sensitization
- ISO 10993-23:2021: Biological evaluation of medical devices - Part 23: Tests for irritation
- ISO 10993-11:2017: Biological evaluation of medical devices - Part 11: Tests for systemic toxicity
- ISO 18562 Series: Biological evaluation of respiratory gas pathways
Toxicological Evaluation:
- ISO 10993-17:2023: Toxicological risk assessment of medical device constituents
Chemical Characterization:
- ISO 10993-18:2020/Amd 1:2022: Determination of the uncertainty factor
Shelf Life & Packaging and Transportation:
- ASTM F1980 / ASTM F88/F88M-15 / ASTM F1140/F1140M-13 / ASTM F1929-15 / ASTM F2096 / ASTM F1886/F1886M / ISO 11607-1: Sterile medical device packaging validation
- ISTA Series / ASTM D 4169: Packaging and transportation testing
Sterilization Validation:
- GB 18280.2: Radiation sterilization dose setting
- ISO 10993-7: Ethylene oxide sterilization residuals
- AAMI TIR30 / AAMI TIR 12 / ASTM F3208-20 / ISO 17664 / ISO 20857 / ISO 25424: Cleaning, disinfection, and sterilization verification
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
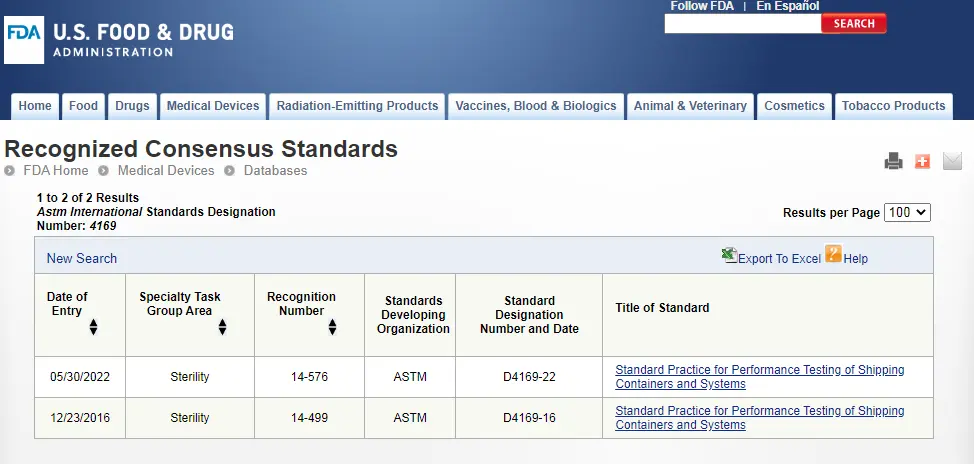 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




