
Transport Simulation Testing for Medical Device Packaging
Simulated Transportation Testing Standards for Sterile Medical Device Packaging
Final Simulated Transportation Testing Standards for Sterile Medical Device Packaging
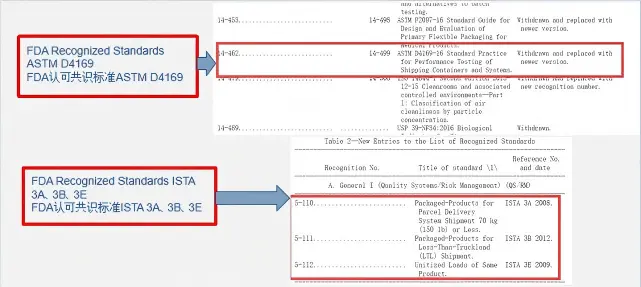
1. FDA-recognized transportation testing standards:
- ISTA 3A
- ISTA 3B
- ISTA 3E
2. Recommended standards for packaging transportation verification under GB/T 19633.1 (Packaging for terminally sterilized medical devices):
- GB/T 4857.17
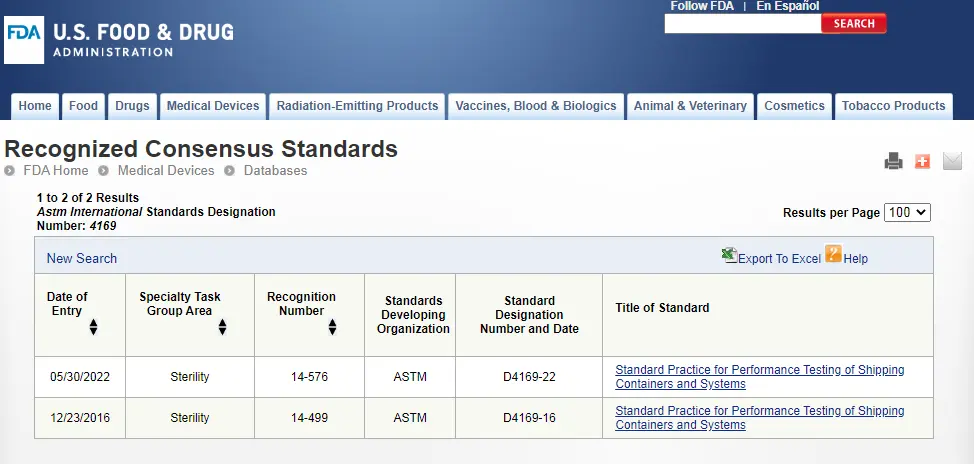
- ASTM D4169
- ISTA Series 1, 2, and 3
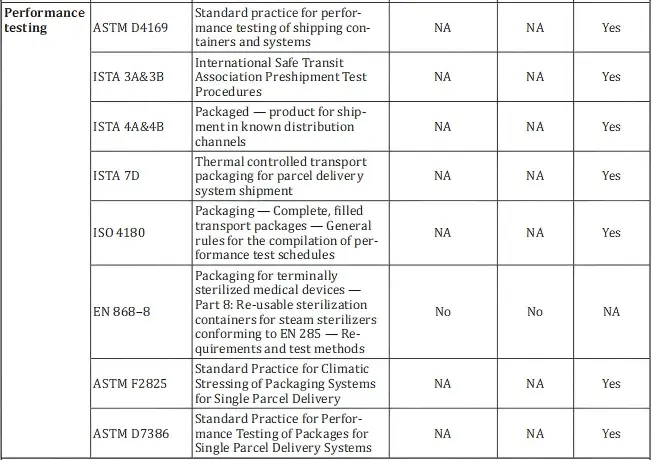
3. Recommended standards under ISO 11607-1:
- ASTM D4169
- ISTA 3A & 3B
- ISTA 4A & 4B
- ISTA 7D
- ISO 4180
4. Standard in YY/T 0681 for sterile medical device packaging:
- YY/T 0681.15
5. Recommendations for Standard Selection:
- For domestic registration:
Prioritize YY/T 0681.15 and GB/T 4857.
Secondary options: ASTM D4169, ISTA 3A, ISTA 3B, ISTA 3E.
- For FDA and CE registration:
Recommended standards: ASTM D4169, ISTA 3A, ISTA 3B, ISTA 3E.
- For both domestic and FDA/CE registration:
Prioritize ASTM D4169.
Secondary options: ISTA 3A, ISTA 3B, ISTA 3E.
- For packaging validation:
Recommended: YY/T 0681.15, ASTM D4169, GB/T 4857.
> Note: YY/T 0681.15 refers to DC13 in ASTM D4169-16, considered the most stringent challenge representative of the domestic circulation of sterile medical device packaging.
6. Scope of Application for Each Standard:
- ISTA 3A (≤70kg):
Applicable to individual product packaging (single parcel), suitable for courier/parcel transportation.
- ISTA 3B:
Applicable to any form of packaging, suitable for LTL (Less-Than-Truckload) transportation.
- ISTA 3E:
Applicable to aggregated product packaging, suitable for any transportation form.
- ASTM D4169:
Covers 18 types of packaging and transportation methods, generally applicable to all commonly used scenarios.
- YY/T 0681.15:
Specifically for sterile medical device packaging.
In the global market, transportation testing of medical device packaging is not only a regulatory obligation but also a vital process to ensure product safety and efficacy. A scientifically sound simulated transportation validation is essential for quality control throughout the product lifecycle.
China JJR Laboratory, as a professional third-party testing organization, provides standardized testing services across multiple regulatory frameworks. We help companies meet domestic and international market entry requirements, reduce circulation risks, and accelerate the compliance process.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




