
The Compliance Process of UDI with the U.S. FDA
On September 24, 2013, the FDA established the Unique Device Identification (UDI) system regulation for medical devices. Manufacturers of medical devices exported to the United States are required to apply for product UDIs within a specified timeframe. The packaging and labeling of devices must bear UDI codes and be uploaded to the GUDID database (one of the important modules of the FDA database). Most of the information submitted to GUDID can also be accessed by the public through Access GUDID, enabling healthcare providers and patients to obtain useful safety information about specific device models.
Next, JJRLAB will provide a detailed introduction on how medical device manufacturers should apply for UDI compliance and enter it into the GUDID database.
I. Introduction to UDI
UDI: Unique Device Identifier is an identity marker assigned to medical devices throughout their entire lifecycle, serving as their unique "identity card" in the product supply chain.
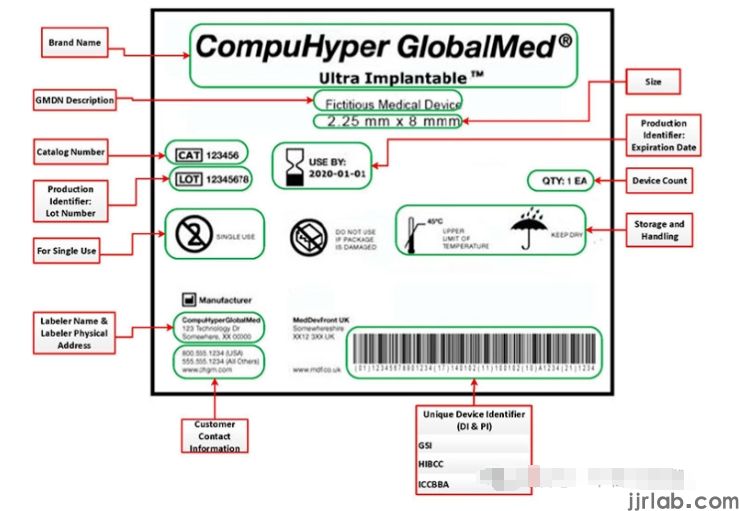
Applicable Formats of UDI
UDI is a code composed of numbers or letters, appearing on the label, packaging, or device itself.
- Device Identifier (DI): A fixed content used to identify the labeler and the device model.
- Production Identifier (PI): Variable content, may include the device's manufacturing batch number, manufacturing date, serial number, expiration date, and unique identification code related to human cells and tissues. Note: UDI for Class I devices does not need to include PI. For devices other than Class I, if the device label contains one or more PIs specified in 21 CFR 801.3, the UDI must include each PI appearing on the label.
Applicable Forms of UDI
Device labels must provide UDI in two forms on both labels and packaging:
1. Human-readable plain text form
2. Automatic Identification and Data Capture (AIDC) technology: Any technology that transmits UDI or device identifiers in a form that can be input into electronic medical records or other computer systems through automated processes. Additionally, the format for labeling dates on device labels and packaging must comply with international standards and conventions (YYYY-MM-DD).
II. Introduction to GUDID
GUDID (Global Unique Device Identification Database) is a global UDI database established by the FDA. GUDID collects and stores critical information about medical devices identified through the UDI system. The database aims to enhance the information tracking capabilities of medical devices, ensure device safety, and promote transparency and regulatory efficiency in the medical device market. The FDA requires manufacturers to submit their medical device UDIs and related product information to GUDID for access by regulatory agencies and the public.
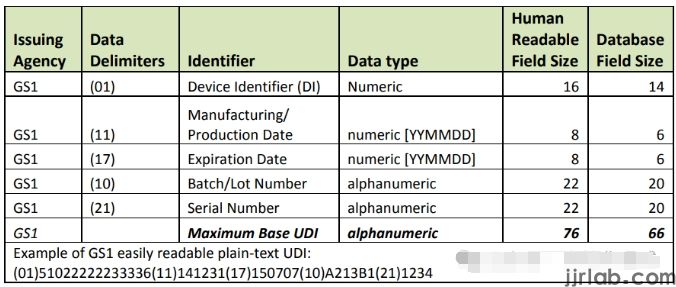
III. Approximate Process of Applying for UDI and Entering it into the GUDID Database:
1. Select an FDA-recognized issuing agency and obtain UDI from the selected issuing agency.
2. Apply for a DUNS and GMDN code.
3. Enter data into the GUDID database.
1. Selecting an FDA-recognized issuing agency
FDA currently authorizes three major organizations to issue UDIs. Each issuing agency has a unique UDI format, which is reviewed and approved by the FDA as part of its accreditation process. Any changes to the UDI format by issuing agencies must be approved by the FDA before implementation.
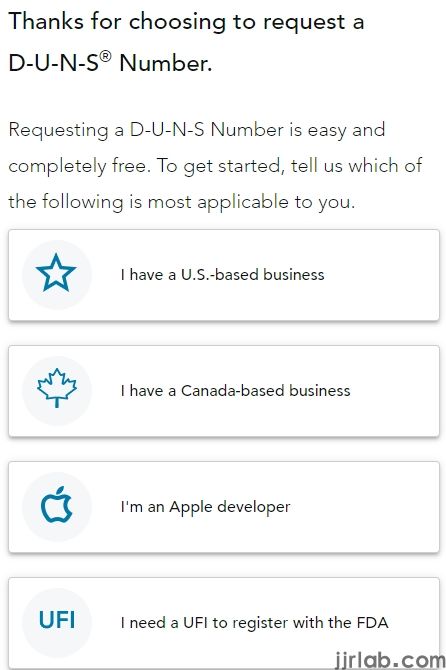
2. Applying for a DUNS code
The Data Universal Numbering System (DUNS) Number is a 9-digit dynamic enterprise identification number. It is widely used for enterprise identification, organization, and compilation of business information. DUNS codes are a prerequisite for FDA registration and certification for medical device companies.
3. Entering data into the GUDID database
To ensure UDI compliance and improve post-market surveillance, the FDA has established the Global UDI Database (GUDID Database), which is accessible to the public to obtain information about corresponding devices. The public can directly input the Device Identifier (DI) from packaging label information into the database webpage to find product information, as well as search through corresponding fields (such as company or product names, common names, or device models and versions).
IV. Benefits of Using UDI
The comprehensive implementation of the Unique Device Identification (UDI) system provides a range of benefits to the industry, FDA, consumers, healthcare providers, and healthcare systems:
1. More accurate reporting, review, and analysis of adverse event reports enable faster identification and correction of issues with medical devices sold and used in the U.S. market, providing a powerful tool for post-market surveillance and recalls.
2. Reduced medical errors by enabling healthcare professionals and others to identify devices more quickly and accurately, obtaining critical information about device characteristics.
3. Standard and clear methods for recording device usage in electronic health records, clinical information systems, claims data sources, and registration records.
4. Enhanced analysis of devices on the market, leveraging a stronger post-market surveillance system to support pre-market approval or clearance of new devices and new uses for currently marketed devices.
5. Providing a foundation for global secure distribution chains, helping to address counterfeit and diverted products, and preparing for medical emergencies. Leading the development of medical device identification systems and gaining recognition worldwide.
JJRLAB is a "one-stop" medical device quality service platform integrating medical device product testing, certification, technical regulatory consulting, and clinical validation. With over twenty years of experience and technological innovation, JJRLAB has a reputation and strength in providing fast and accurate assistance to medical device companies in applying for UDI compliance and entering it into the GUDID database, as well as providing one-stop services for medical device FDA registration and certification, helping companies smoothly enter the U.S. market.
Email:hello@jjrlab.com
Write your message here and send it to us
 JJRLAB's New Chemical Laboratory Expansion Complet
JJRLAB's New Chemical Laboratory Expansion Complet
 JJRLAB 5G NR and Sub 6G communication testing
JJRLAB 5G NR and Sub 6G communication testing
 JJRLAB Completes Expansion of CPSC Full Project
JJRLAB Completes Expansion of CPSC Full Project
 What certifications for Middle East exports?
What certifications for Middle East exports?
 US-bound button cell batteries need UL4200A-2023
US-bound button cell batteries need UL4200A-2023
 Button batteries ANSI/UL 4200A-2023 Laboratory
Button batteries ANSI/UL 4200A-2023 Laboratory
 What is a Dun & Bradstreet (D-U-N-S®) Number?
What is a Dun & Bradstreet (D-U-N-S®) Number?
 The Compliance Process of UDI with the U.S. FDA
The Compliance Process of UDI with the U.S. FDA
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




