
TEMU's European Platform Requires DoC Compliance Statement
Recently, TEMU has issued a new regulation requiring that products under CE control must not only upload CE certification documents but also provide DoC or CoC compliance statement materials. For non-compliant products, TEMU will issue risk warnings to buyers, delist the products, notify buyers or other relevant parties to destroy/return the products, etc. Previously, Amazon also required relevant sellers to submit EU doc compliance statements; otherwise, product links would be restricted and delisted.
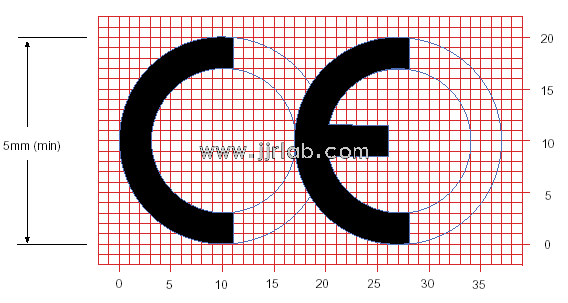
What is a DoC Compliance Document?
A DoC (Declaration of Conformity) compliance document is a mandatory file that serves as a self-declaration model within CE certification, stating that the product meets all relevant safety requirements before entering the European market.
By drafting and signing the Declaration of Conformity, the manufacturer or brand assumes responsibility for the product's compliance. The significance of the DoC compliance statement lies in the manufacturer's or brand's full responsibility for ensuring their product meets EU compliance standards. This document is a self-declaration.
Currently, many e-commerce platforms require sellers to provide the EU Declaration of Conformity when uploading product compliance documents.
Which Products Require a DoC Compliance Document?
Typically, in the EU market, products that need the CE safety certification mark require a Declaration of Conformity.
These products must pass tests, ensuring complete technical documentation for product safety. Only after obtaining CE certification can they be manufactured, sold, and circulated in the EU market.
For sellers, the following products must provide a compliance statement to be sold:
- Electronic products
- Electronic toys
- Batteries/power supplies
- Chargers
- Kitchen equipment
- Sunglasses
- Mechanical fitness equipment
- Lighting fixtures
- Cosmetics
For more detailed information on other products, please refer to the EU website:https://single-market-economy.ec.europa.eu/single-market/european-standards/harmonised-standards_en
How to Ensure DoC Compliance?
For imported products, the importer must ensure the product comes with a DoC compliance statement and must keep a copy for 10 years after the product is marketed (unless otherwise specified by product-related CE directives).

It is important to note that one product can have a single DoC, which can cover all sales models of that product.
The EU Declaration of Conformity must be issued and signed by the product's manufacturer or importer, not by a notified body or testing organization. When importing products from non-EU countries/regions, the importer is responsible for ensuring the product meets all legal requirements and that the technical documentation (including the declaration) is correct and available.
Besides the manufacturer, the Declaration of Conformity can also be signed by the manufacturer's authorized representative (a natural or legal person established within the European Economic Area).
What Information Should Be Included in a DoC Compliance Statement?
A DoC compliance statement must include the following information:
- Manufacturer's information: name, address, etc.
- Product information: product name, brand, model, etc.
- Relevant product safety report directives and information in the safety report (which should correspond to the information in the DoC).
- Compliance declaration
- Authorized representative’s information: name, address, etc.
- Responsible person's signature
- Date of issuance of the declaration
Can a CE Certificate or Test Report Replace the DoC Compliance Statement?
No, it cannot. While the DoC compliance statement is just a compliance document, it is as important as CE certification and cannot be substituted. Products manufactured, sold, and circulated in the EU market with the CE safety certification mark usually require a Declaration of Conformity.
Email:hello@jjrlab.com
Write your message here and send it to us
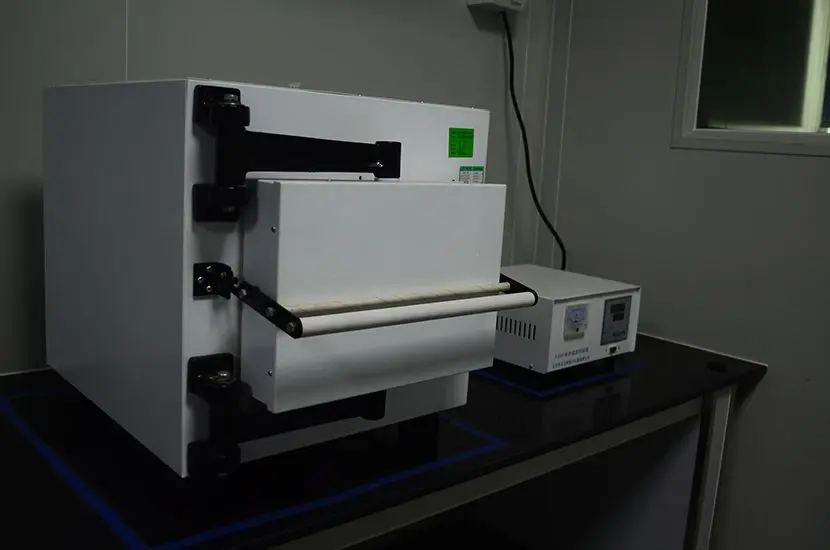
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
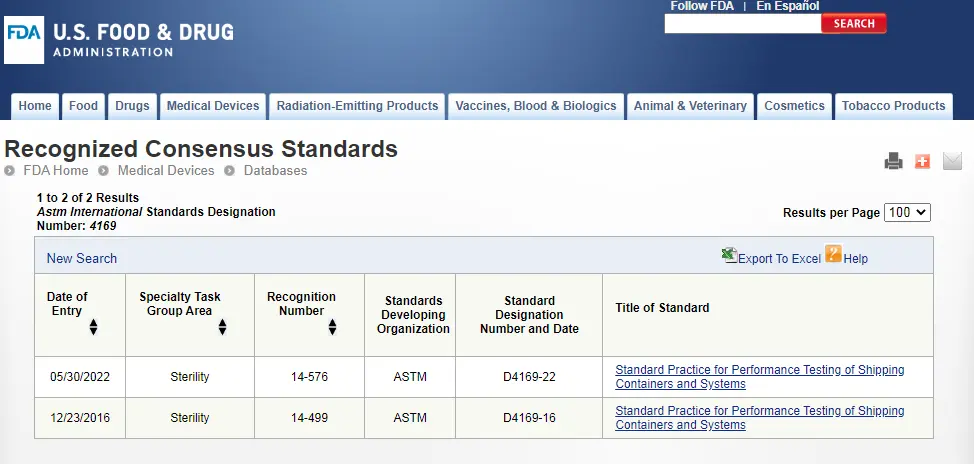
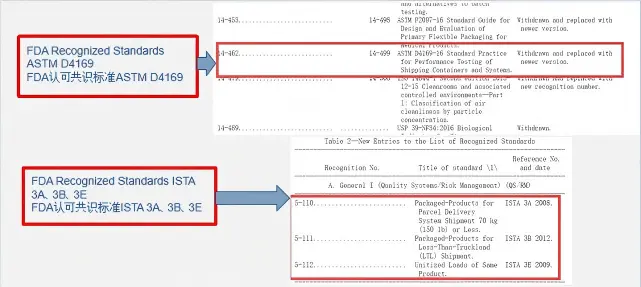
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




