
Surgical Medical Devices GB 9706 Testing
What are surgical medical instruments?
Surgical medical instruments refer to tools used in surgical operations for diagnosing, treating, preventing diseases, or dissecting tissues. These instruments include scalpels, scissors, hemostats, retractors, probes, needle holders, endoscopes, etc., which are used for cutting, clamping, retracting, probing, suturing, and fixing tissues, ensuring the smooth progress of surgery.
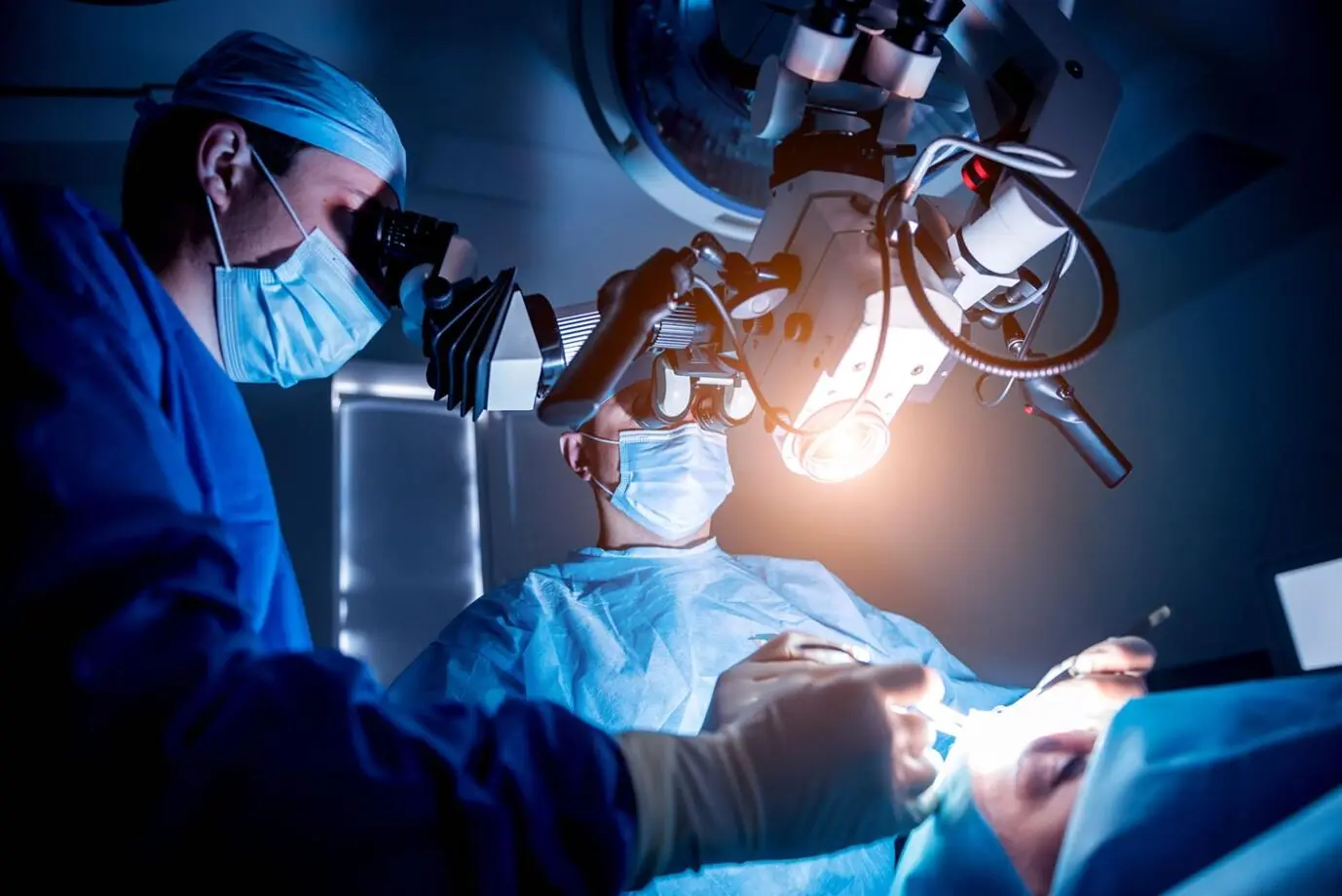
What are some common surgical medical instruments?
Common surgical medical instruments include scalpels and scissors (used for cutting), hemostats (used for stopping bleeding), needle holders and suturing needles (used for suturing), endoscopes (used for observing internal structures), as well as suction devices and electrosurgical units (used for suctioning fluids and stopping bleeding). These instruments come in various sizes and models depending on the type and needs of the surgery.
What testing standards must surgical medical equipment meet to enter China?
- GB 9706.202-2021 Medical electrical equipment—Part 2-2: Particular requirements for the basic safety and essential performance of high frequency surgical equipment and high-frequency accessories
GB 9706.202 applies to the basic safety and essential performance of high-frequency surgical equipment and high-frequency accessories.
- GB 9706.204-2022 Medical electrical equipment—Part 2-4: Particular requirements for the basic safety and essential performance of cardiac defibrillators
- YY 9706.210-2021 Medical electrical equipment—Part 2-10: Particular requirements for the basic safety and essential performance of nerve and muscle stimulators
YY 9706.210 specifies safety requirements for nerve and muscle stimulators used in physical medicine practices, including transcutaneous electrical nerve stimulators and electrical muscle stimulators. Note: Muscle stimulators may also be considered neuromuscular stimulators.
YY 9706.210 does not apply to the following devices: devices for implantation or those connected to implanted electrodes; devices used for brain stimulation (e.g., electroconvulsive therapy devices); devices used for neurological research; external cardiac pacemakers; devices for evoked response diagnosis; electromyography devices; and cardiac defibrillation devices.
- GB 9706.212-2020 Medical electrical equipment—Part 2-12: Particular requirements for basic safety and essential performance of critical care ventilators
GB 9706.212 specifies the most basic requirements for the design and manufacture (including structure, operating modes, gas connections, signal waveforms, alarms, accompanying documents, accessories, labels, and packaging, etc.) of therapeutic ventilators to ensure that when used correctly according to the manufacturer's intended purpose, in the intended environment and conditions, no harm or danger will occur to users, patients, or the environment.
GB 9706.212 does not apply to other specialized ventilators (e.g., sleep apnea ventilators or emergency transport ventilators).
- GB 9706.213-2021 Medical electrical equipment—Part 2-13: Particular requirements for the basics safety and essential performance of an anesthetic workstation
GB 9706.213 specifies particular requirements for a complete anesthetic workstation and the following anesthetic workstation components.
GB 9706.213 applies to anesthetic workstations that require continuous participation by professional operators, used for the administration of inhalation anesthesia.
- GB 9706.218-2021 Medical electrical equipment—Part 2-18: Particular requirements for the basic safety and essential performance of endoscopic equipment
JJR Laboratory in China is a compliant laboratory located in China, providing GB 9706 testing and assisting you with CFDA (NMPA certification) applications.
Email:hello@jjrlab.com
Write your message here and send it to us
 When Can FCC ID Modifications Be Filed?
When Can FCC ID Modifications Be Filed?
 LoRa Certification Testing Laboratory
LoRa Certification Testing Laboratory
 Blood Pressure Monitor Certification Testing Servi
Blood Pressure Monitor Certification Testing Servi
 ECG Device Certification Testing
ECG Device Certification Testing
 Pulse Oximeter Certification and Testing Standards
Pulse Oximeter Certification and Testing Standards
 IVD Medical Device GB 4793:2024 Test Report
IVD Medical Device GB 4793:2024 Test Report
 IECEE CBTL Testing Laboratory for IVD Medical Devi
IECEE CBTL Testing Laboratory for IVD Medical Devi
 China OECD GLP-Certified Laboratory
China OECD GLP-Certified Laboratory
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




