
MRI Compatibility Testing According to ASTM F2052
In a magnetic resonance (MR) environment, the magnetic resonance phenomena generated by MRI equipment can pose certain risks to patients and personnel within the area. Therefore, medical device manufacturers should consider the safety and effectiveness of implants or medical devices expected to enter MR environments as part of their risk management procedures.
Previously, we outlined the safety markings for MRI compatibility. Based on ASTM F2503 and YY/T 0987.1, these markings are categorized into three types: MR Safe, MR Unsafe, and MR Conditional. Additionally, a fourth label, “Safety in MRI Not Evaluated,” is introduced in FDA guidelines, which can be used under specific circumstances.
Relevant Guidelines/Testing Standards:
- Guideline for Testing and Labeling Medical Devices for Safety in the Magnetic Resonance (MR) Environment
- ASTM F2052 Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment
- YY/T 0987.2-2016 - MRI Compatibility of Surgical Implants Part 2: Magnetically Induced Displacement Force Testing Method
Definitions
1. Magnetically Induced Displacement Force: The force produced when a magnetic object is exposed to the spatial gradient of a magnetic field. This force causes the object to move in the gradient field.
2. Diamagnetic Material: A material whose relative permeability is less than unity.
- Note: Magnetic permeability refers to a material's physical property that indicates how easily it can be magnetized.
3. Ferromagnetic Material: A material whose magnetic moments are ordered and parallel, producing magnetization in one direction.
4. Paramagnetic Material: A material with a relative permeability slightly greater than unity and which is practically independent of the magnetizing force.
- Note: Many common medical device materials are paramagnetic, including titanium and titanium alloys, stainless steel 316L, nickel-titanium (non-magnetic nickel and titanium alloy), and CoCrMo alloys.
Basic Principle of Testing Method
The displacement force is produced when ferromagnetic, paramagnetic, and diamagnetic materials are exposed to the spatial gradient of a static magnetic field.
Evaluation Criteria
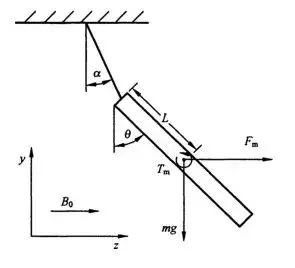
For devices that are safe in the MR environment, the magnetically induced displacement force should be less than the gravitational force acting on the device (its weight), i.e., the angle of displacement (α) should be less than 45°.
- Note: This is a reference point but not an absolute acceptance criterion. ASTM F2052-2015 states that if the magnetically induced displacement force exceeds gravity, the device’s position and fixation methods must be considered and demonstrated under specific conditions.
Testing Method
Equipment:
- A system where the displacement force direction is horizontal.
- Non-magnetic support.
- A protractor with a minimum scale of 1°.
Note: MR systems are categorized into open and cylindrical types. Open systems have higher spatial gradients in the static magnetic field, which may produce stronger displacement forces.
Samples:
- Medical devices that can be suspended, with the weight of the suspension line not exceeding 1% of the device's weight.
- Note: This test standard is not applicable to devices that are too large to be suspended.
- The test must be performed on finished products, which have undergone final processing (e.g., sterilization).
- Note: According to U.S. guidelines, if a medical device is composed of paramagnetic materials that are unaffected by processing and manufacturing, test results from samples made from the same materials can be used. However, products made from alloys such as nitinol and austenitic stainless steels 303 and 304, which are affected by manufacturing processes, should be tested as finished products.
For medical devices of multiple sizes, the device with the largest magnetic material mass or the highest proportion of magnetic material by weight should be used to assess the worst-case scenario for magnetically induced displacement force.
Important Notes:
1. Each test sample should be tested at least three times.
2. If equipment is bundled during testing, the total weight of the bundled equipment should not exceed 1% of the test device's weight.
3. For devices containing electrical wires or cables, adjust the device to minimize the effect of the wires or cables on the measurements.
Email:hello@jjrlab.com
Write your message here and send it to us
 Amazon UL Standard Test Report
Amazon UL Standard Test Report
 When Can FCC ID Modifications Be Filed?
When Can FCC ID Modifications Be Filed?
 LoRa Certification Testing Laboratory
LoRa Certification Testing Laboratory
 Blood Pressure Monitor Certification Testing Servi
Blood Pressure Monitor Certification Testing Servi
 ECG Device Certification Testing
ECG Device Certification Testing
 Pulse Oximeter Certification and Testing Standards
Pulse Oximeter Certification and Testing Standards
 IVD Medical Device GB 4793:2024 Test Report
IVD Medical Device GB 4793:2024 Test Report
 IECEE CBTL Testing Laboratory for IVD Medical Devi
IECEE CBTL Testing Laboratory for IVD Medical Devi
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




