
Medical Device Safety Testing under GB 9706.1-2020
The testing items for medical device safety compliance include the following:
1. Structural inspection and testing: Ensure that the structure of the device meets standard requirements, including the connection, fixation, and protection of various components.
2. Power supply voltage adaptation: Test whether the device can operate normally under specified power supply voltages within the defined range.
3. Insulation resistance: Verify if the insulation resistance of the device's materials meets standard requirements to prevent risks such as electric leakage and shock.
4. Leakage current: Test whether the current on the device's casing or external components meets standard requirements during normal operation, ensuring user safety.
5. Dielectric strength (high voltage test): Determine if the insulation materials of the device can withstand specified electric field strengths and durations without breakdown or abnormal conditions.
6. Abnormal operation and fault conditions: Assess whether the device can safely shut down and alert operators for maintenance under abnormal operational or fault conditions.
7. Mechanical strength: Evaluate if the device's structure can withstand specified external forces, mitigating safety risks from mechanical damage.
8. Stability: Check if the device can maintain stability under normal operating conditions without significant shaking or displacement.
9. Liquid ingress: Verify if the device can prevent liquid from entering its internals to avoid issues such as circuit short-circuiting.
10. Cleaning and disinfection: Assess if the device can be easily cleaned and disinfected to maintain cleanliness and hygiene.
11. Temperature and humidity: Evaluate if the device can operate normally within specified temperature and humidity ranges, preventing safety risks due to unsuitable conditions.
12. Grounding test: Ensure that the device's grounding meets standard requirements to prevent safety risks related to electric leakage.
China JJR Laboratory holds CNAS and CMA certification and is accredited under IEC 17025 and GLP. We offer cost-effective testing services, potentially saving you 30% on certification testing costs. Welcome to submit your samples for testing.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
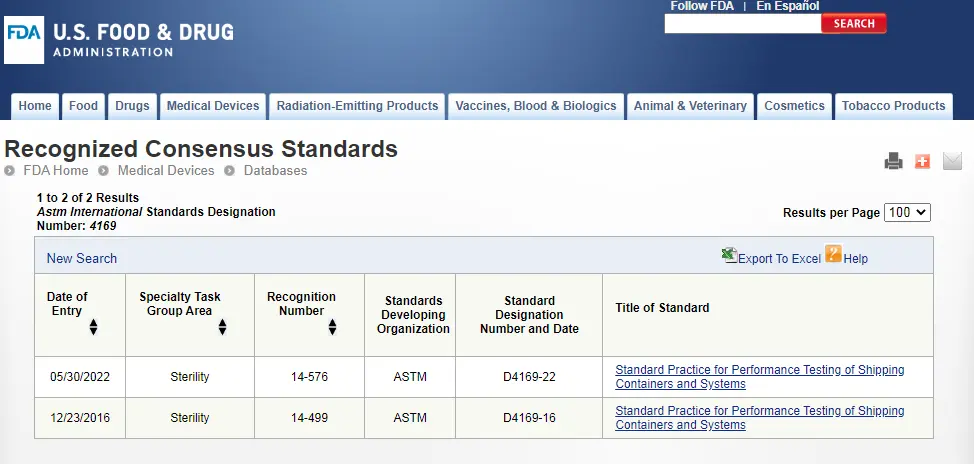 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
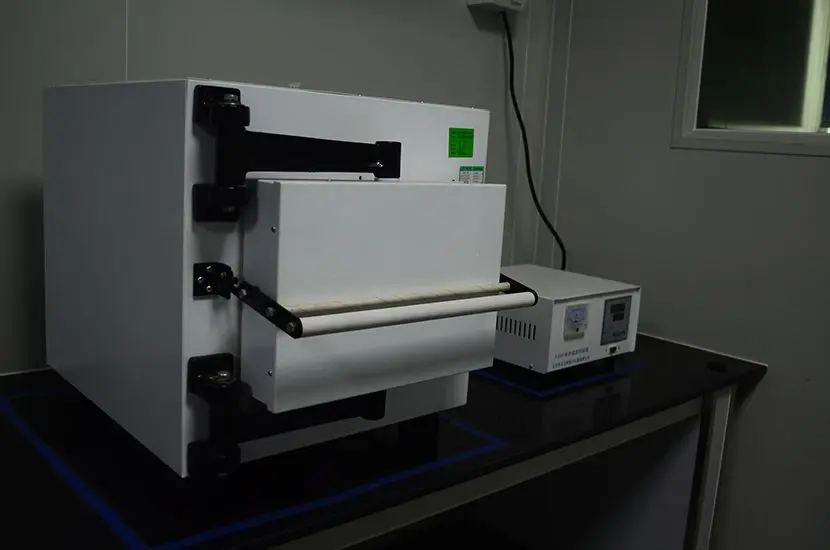 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
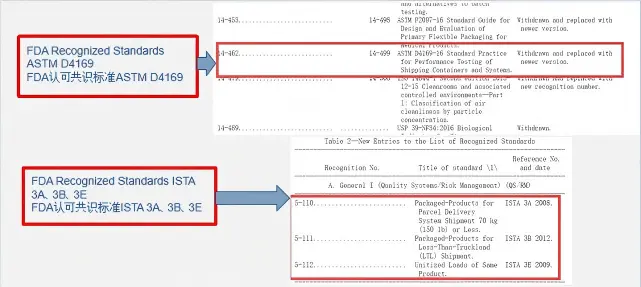 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
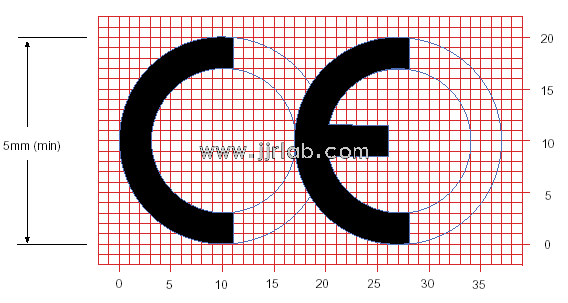 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




