
Medical Device Electrical Safety Testing
IEC 60601 is a harmonized standard used to evaluate the electrical safety and effectiveness of medical devices and equipment. Recognized by medical device regulators in most global markets, the latest version is IEC 60601 3rd edition, amendment 1.
In markets such as the United States, Europe, and Australia, medical device regulators require devices to undergo electromagnetic compatibility (EMC) testing. This ensures that devices possess adequate safety and performance characteristics. Specifically, regulators mandate EMC testing to determine if a device can operate safely and effectively in its intended environment without causing electromagnetic interference.
Medical Device Electrical Safety Testing Standards:
These standards are typically issued by the International Electrotechnical Commission (IEC). The most common standards include:
- IEC 60601-1: Medical electrical equipment, Part 1: General requirements. This standard specifies general requirements for medical electrical equipment, covering electrical, mechanical, radiation, and chemical safety.
- IEC 60601-1-2: Medical electrical equipment, Part 1-2: Electromagnetic compatibility. This standard sets the EMC requirements to ensure the equipment operates correctly in an electromagnetic interference environment.
- IEC 62353: Medical devices testing and measuring equipment safety. This standard specifies safety testing requirements for testing and measurement equipment used in medical devices, focusing on electrical safety performance and operational safety.
- IEC 61010-1: Safety requirements for electrical equipment for measurement, control, and laboratory use. This standard covers general safety requirements, including electrical, mechanical, thermal, and radiation safety.
- GB 9706.1-2010: Medical electrical equipment, Part 1: General requirements. This Chinese standard adopts IEC 60601-1, detailing general requirements for medical electrical equipment, including electrical, mechanical, radiation, and chemical safety.
It is important to note that the specific content and applicability of these standards may vary by country and region. Therefore, testing and certification should adhere to local laws and regulations.
Additional Standards for Specific Medical Devices:
Apart from the general standards, different types of medical devices may need to comply with other specific standards. For example:
- Electrocardiographs (ECGs) must meet IEC 60601-2-25 requirements.
- Blood glucose meters must meet ISO 15197 requirements.
Electrical Safety Testing Projects for Medical Devices:
Electrical safety is crucial in the medical field to ensure the proper functioning of devices and prevent accidents and injuries caused by electrical faults. Key electrical safety testing projects include:
1. Electrical Parameter Testing:
This involves measuring parameters such as voltage, frequency, current, and power factor to ensure the device's electrical performance meets the relevant standards and regulations. Proper voltage and frequency ensure the device functions correctly in its intended environment.
2. Insulation Testing:
This common test measures the insulation resistance of a device to evaluate its insulation performance. It aims to prevent electrical short circuits or leakage, reducing the risk of electric shock to patients or healthcare workers.
3. Grounding Testing:
This test measures the grounding resistance between the device and the ground to ensure effective grounding. Proper grounding minimizes the risk of leakage current and provides a safe operating environment.
4. Leakage Current Testing:
Leakage current is an important indicator in electrical safety testing. This test assesses whether the device has any leakage current and if it exceeds the safety limits specified by standards. It ensures that leakage current is within safe limits to avoid potential hazards.
5. Reliability Testing:
This evaluates the stability and durability of medical devices over extended use. Tests include subjecting devices to various conditions such as high and low temperatures and humidity. It ensures the device operates reliably in different environmental conditions.
Conclusion:
Electrical safety testing for medical devices includes tests for electrical parameters, insulation, grounding, leakage current, and reliability. These tests aim to ensure the normal operation and safety of medical devices, protecting patients and healthcare workers. Comprehensive testing and evaluation can identify and rectify potential safety issues, enhancing device safety and reliability.
About Us:
JJR Laboratory in China is IEC 17025 accredited and GLP authorized. We can help companies save 30% on certification testing costs. We welcome your testing submissions.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
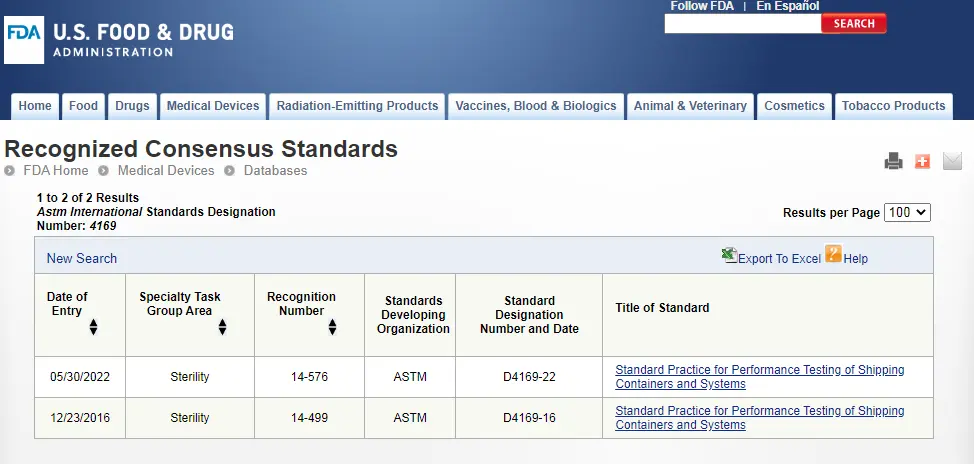 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
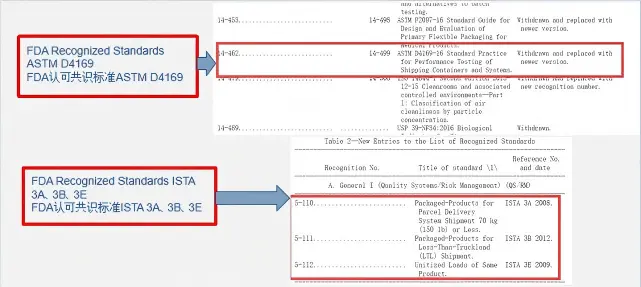
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
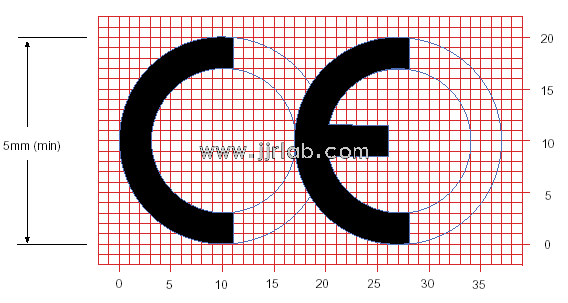 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




