
Medical Device ANVISA Registration in Brazil
The registration of medical devices in Brazil is managed by the Brazilian National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA). The main regulation is RDC 751/2022, among others. Manufacturers located outside Brazil must have a local agent in Brazil to hold the certification.
In Brazil, medical devices are classified into Class I, II, III, and IV based on risk level, ranging from low to high. Manufacturers of all risk class products must comply with the Brazilian Good Manufacturing Practice (BGMP) requirements.
For lower risk Class I and II medical devices, BGMP certification is not required for registration. Manufacturers only need to have a quality management system compliant with BGMP requirements. ANVISA does not audit technical documents for these classes, but technical files must be maintained by a Brazilian agent for ANVISA inspection purposes.
Higher risk Class III and IV medical devices must obtain BGMP certification before submitting technical files for ANVISA review. It's important to note that although Brazil participates in the Medical Device Single Audit Program (MDSAP), MDSAP only accelerates the process and cannot substitute BGMP certification.
The requirements for technical documents in Brazil are referenced in RDC 751/2022 regulations. To align with international standards, documents other than labels and instructions can be submitted in Portuguese, Spanish, or English.
The registration fee for low-risk Class I and II products is approximately $500, with a processing time of about one month. For high-risk Class III and IV products, the registration fee is approximately $1500, in addition to around $25,000 for BGMP audit. The total registration time, including BGMP audit, is approximately 1.5 to 2 years.
After successful registration, there is no time limit for Class I and II products, while registration for Class III and IV products is valid for ten years. BGMP certificates are valid for two years, and ANVISA or its accredited third-party agencies conduct audits every two years. Registration certificates can be transferred to new agents, but this process requires cooperation from the previous agent.
JJRLAB in China can assist you with completing product registration in Brazil and provide local agent services. If you would like to learn more about registering your product in Brazil, please feel free to contact us.
Email:hello@jjrlab.com
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
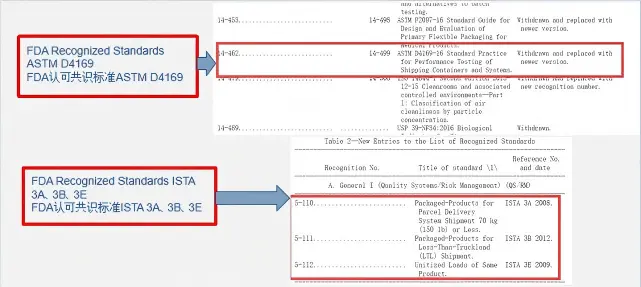 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




