
ISO 10993 Biocompatibility Testing Fees
The cost of ISO 10993 medical device biocompatibility testing is US$4,000, with a cycle of 4 weeks (cytotoxicity testing, sensitization testing, and skin irritation); our laboratory has CNAS and CMA qualification seals, and is a GLP-authorized laboratory that can help you Provide Chinese and English compatibility test reports;
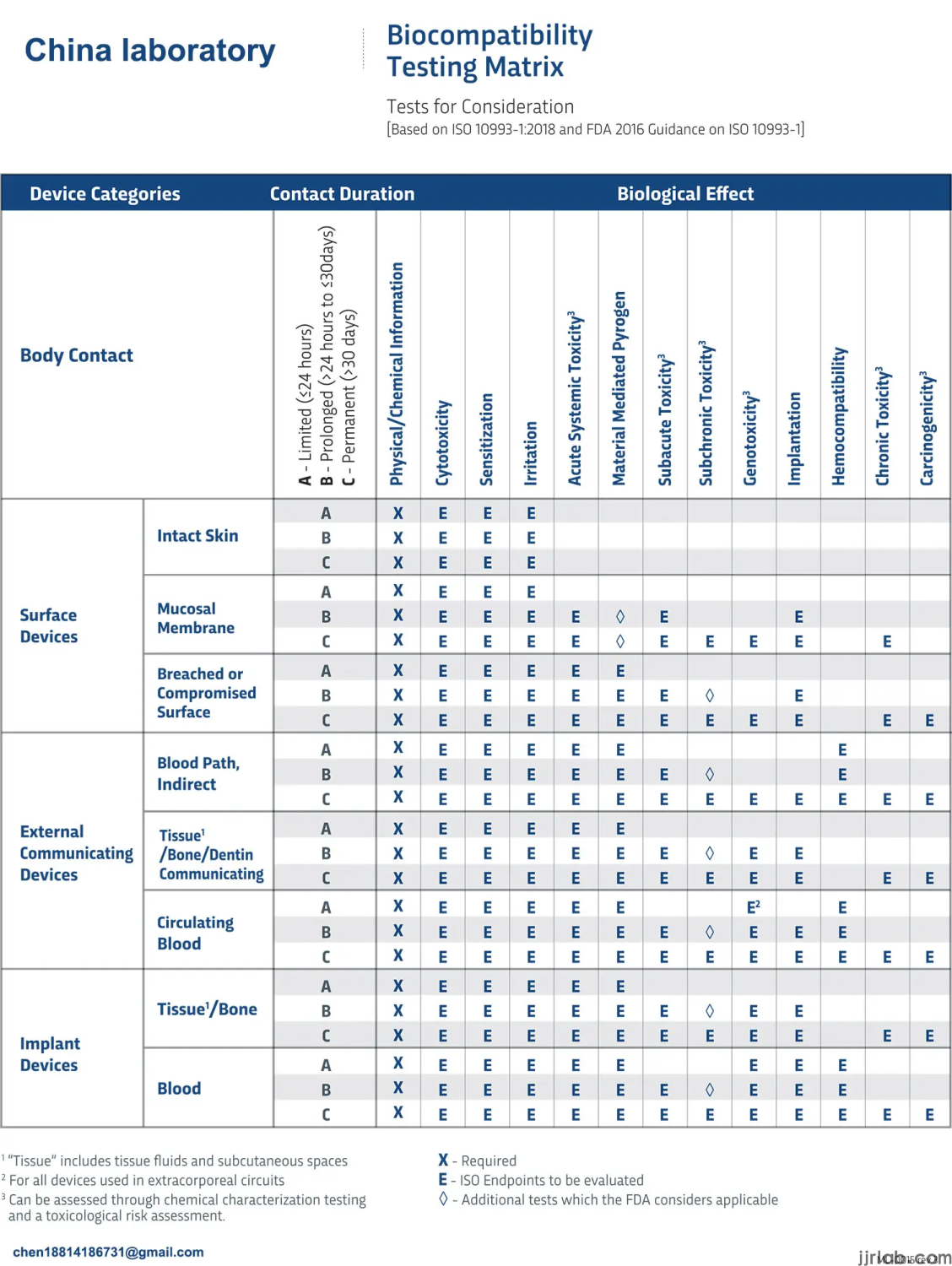
Biocompatibility is a measure of the compatibility of a device or material with a biological system. The ISO 10993-1:2018 standard defines biocompatibility as "the ability of a medical device or material to perform with an appropriate host response in a specific application".
"The main purpose of this part of ISO 10993 is to protect humans from potential risks arising from the use of medical devices." (ISO 10993-1:2018). Manufacturers must better understand device materials, device manufacturing, sterilization, and other processes before conducting biocompatibility testing. The ISO 10993-1:2018 standard emphasizes chemical characterization prior to in vitro and in vivo biocompatibility testing.
Email:chen18814186731@gmail.com

In vitro cytotoxicity – ISO 10993-5
Cytotoxicity is a biocompatibility test performed on mammalian cells in culture. PBL performs three in vitro cytotoxicity tests: MEM elution, agarose overlay, and direct contact.
Equipment extracts and leachables – ISO 10993-18
Typically, chemical characterization and analysis of device components, also known as device extractables and leachables testing, is performed prior to any biological testing. This involves extracting leachable material from a device or component at high temperatures and analyzing the extract using a variety of instruments.

Hemolysis Test - ASTM F756:
We perform hemolysis tests by direct and extraction methods to evaluate the adverse effects of medical devices, leachables, and biological materials on rabbit blood. This assay is ideal for evaluating the blood compatibility of medical devices and biological materials according to international standards ISO 10993-4:2017 and ASTM F756.
In vivo biocompatibility testing:
Following the completion of in vitro testing, in vivo biological testing will be performed, the range of types of testing will depend on the intended use of the device. In vivo testing can range from skin irritation testing to sensitization testing, implant testing and systemic toxicity testing. Test turnaround times can vary from three weeks to several months, depending on the specific test data required. Subchronic or chronic systemic toxicity studies can last longer.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
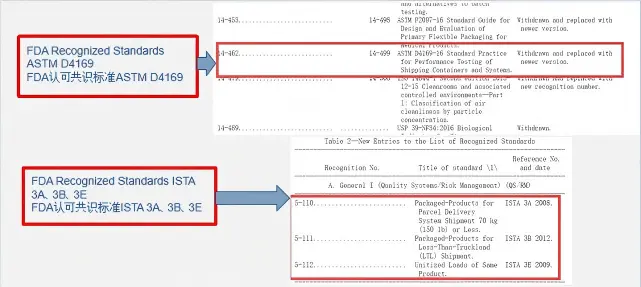
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




