
ISO 10993-5 In Vitro Cytotoxicity Testing
In vitro cytotoxicity testing is an in vitro assay conducted under ex vivo conditions to simulate the biological environment, assessing the potential cytolytic, growth-inhibiting, and other toxic effects of medical devices and biomaterials on tissues following contact.
Following effective evaluations of physical and chemical properties, processing performance, and morphology, medical device manufacturers are required to conduct biocompatibility assessments in accordance with ISO or GB/T standards to minimize the risks associated with medical devices. In vitro cytotoxicity testing stands as one of the most critical metrics in the biological evaluation system of medical devices, mandatory before clinical application of devices such as disposable medical masks, medical sodium hyaluronate dressings, dental implant guides, single-use sterile sampling swabs, disposable surgical gowns, disposable minimally invasive surgical dilators, and medical atomizers, among others, to assess their safety.

Purpose and Significance of Cytotoxicity Testing
Purpose: To assess the potential cytotoxic reactions of medical devices and biomaterials to cells and predict tissue cell responses upon final biological application. Through in vitro cell culture techniques, the assay detects cell growth inhibition, functional changes, cell lysis, cell death, or other toxic reactions upon exposure to the test samples.
Significance: It economically and efficiently screens cytotoxicity of batch samples within a short period, providing a prerequisite for whether animal testing should proceed and ensuring crucial validation for the development and application of new medical devices and biomaterials.
Testing Basis
GB/T 16886.5-2017 Biological Evaluation of Medical Devices—Part 5: Tests for in vitro cytotoxicity ISO 10993-5:2009 Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity GB/T 14233.2-2005 Inspection methods for infusion, transfusion, and injection equipment—Part 2: Biological test methods YY/T 0127.9-2009 Biological evaluation of oral medical devices—Part 2, Unit 9: Test methods—Cytotoxicity testing: agar diffusion method and membrane diffusion method
Introduction and Selection of Cytotoxicity Testing Methods
In vitro cytotoxicity testing is versatile and applicable to various medical devices and biomaterial evaluations, categorized mainly into extract tests, direct contact tests, and indirect contact tests. The selection of the testing method depends on the material of the medical device and its interaction pathways with the organism. When multiple test methods are applicable, factors such as principles, sensitivity, optionality, quantifiability, reproducibility, suitability of test materials, and limitations should be considered for accurate method selection.
Steps of In Vitro Cytotoxicity Testing
Cell Seeding Plate: Cells are suspended by digesting them with trypsin in a culture bottle. After centrifugation at 3000 rpm for 3 minutes, the supernatant is removed. Cell suspension at the specified concentration is added to 96-well plates, 6-well plates, or culture dishes and placed in a CO2 incubator for the required duration according to standards.
Sample Extraction and Medium Change: Sterile samples are prepared under aseptic conditions and immersed in extraction media at 37°C for 24 or 72 hours as per experimental protocols. Blank controls, negative controls, and positive controls are prepared concurrently according to standards. After extraction, the cell culture medium is replaced with fresh medium following standard procedures.
Observation of Results: Various methods such as MTT assay, direct contact method, and indirect contact method are employed to observe cell morphology and determine cytotoxicity according to established criteria.
Reasons for Occurrence of Cytotoxicity in Products
1) Characteristics of raw materials
2) Presence of evident cytotoxicity in products such as drug-coated catheters or coated guide wires
3) Suitability of selected testing methods for the product; gel-based, paste-like, or colored liquid products may not be suitable for extraction methods
4) Changes in pH due to exposure to extraction media
5) Handling of products with pre-flush solutions
6) Residual amounts after ethylene oxide sterilization
The presence of cytotoxicity in sample cells does not necessarily preclude clinical application. Results should be interpreted in conjunction with other test data, including physical and chemical characterization of materials, product composition, and intended use. Further evaluation may be necessary to clarify cytotoxic results.
References
GB/T 16886.5-2017 Biological Evaluation of Medical Devices—Part 5: Tests for in vitro cytotoxicity ISO 10993-5:2009 Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity GB/T 14233.2-2005 Inspection methods for infusion, transfusion, and injection equipment—Part 2: Biological test methods
YY/T 0127.9-2009 Biological evaluation of oral medical devices—Part 2, Unit 9: Test methods—Cytotoxicity testing: agar diffusion method and membrane diffusion method
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
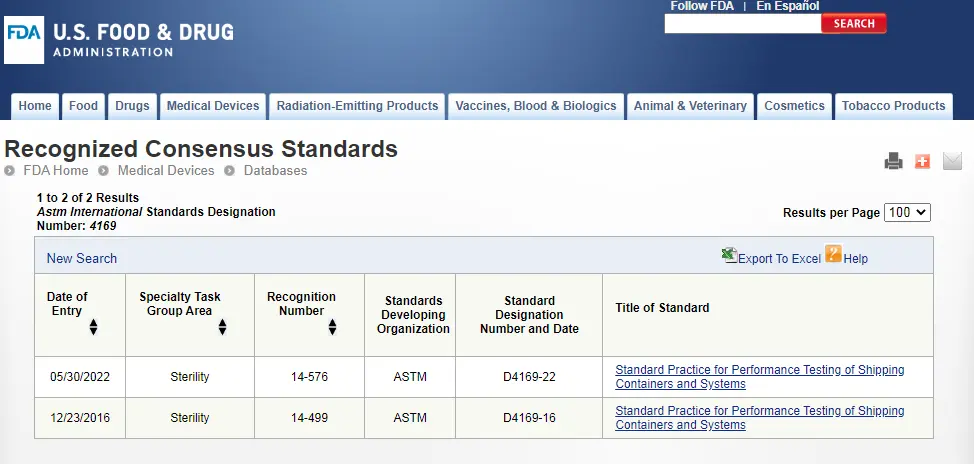 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
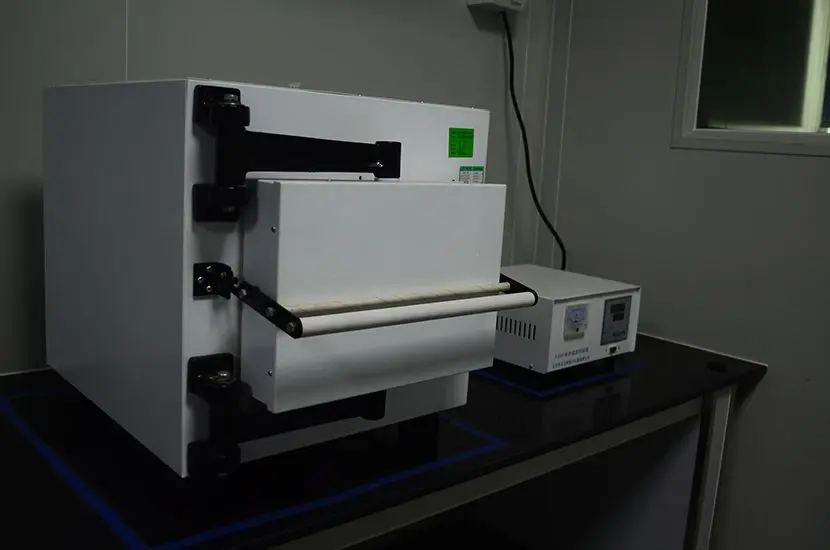 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
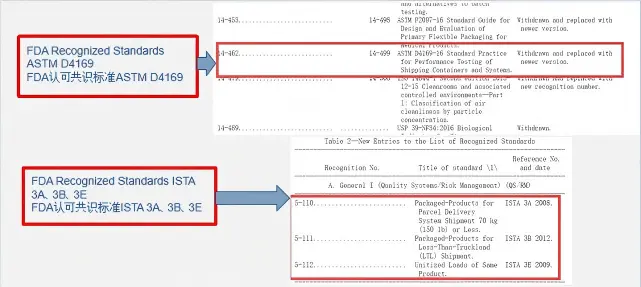 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




