
ISO 10993-17:2023 Testing Laboratory
The release of the updated ISO 10993-17:2023 standard marks a shift in medical device biocompatibility toward emphasizing the toxicological risk assessment (TRA) process. The new standard specifies that after completing material cheMICal characterization of medical devices (ISO 10993-18), a toxicological risk assessment shoULd be conducted following the guidelines in iso 10993-17 to determine the biological safety of the device.
Additionally, compaRED to ISO 10993-17:2002, the updated standard introduces several key concepts, including TSL, EEDmax, and MoS.
Toxicological Assessment Process
The toxicological assessment of medical devices is a systematic process designed to ensure the safety of devices for human use. It primarily involves three core steps:
1. Hazard Identification
2. Exposure Estimation
3. Risk Evaluation
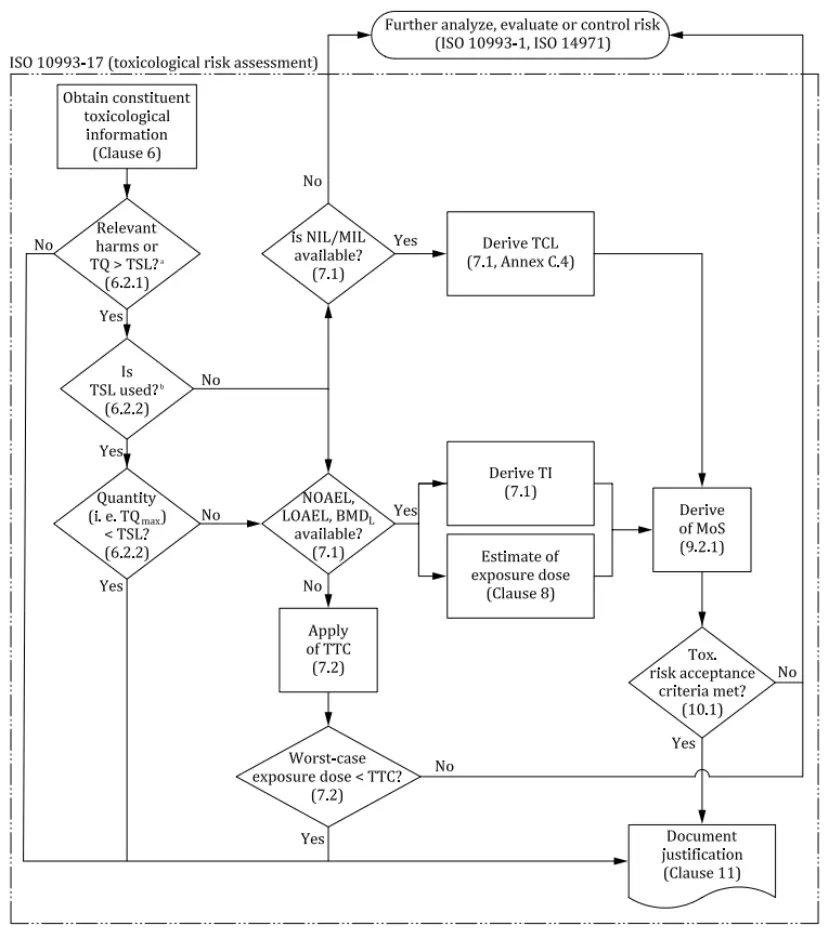
Each step incorporates specific technical and computational methods. This entire process requires interdisciplinary collaboration, bringing together chemists, toxicologists, biomedical engineers, and clinical experts. Through this process, the safety of medical devices can be ensured, meeting relevant regulatory requirements.
New Key Concepts
TSL (Toxicological Screening Limit)
TSL is the highest cumulative exposure dose for a specific substance within a defined period, set to protect individuals from health hazards.
According to ISO 10993-17, if the total quantity (TQ) of a compound extractable from a medical device within a given time frame is below the specified TSL, the exposure level can be considered toxicologically insignificant, and further toxicological risk assessment is typically unnecessary.
Applicable Scenarios for TSL:
1. Systemic toxicity (e.g., acute, subacute, chronic, subchronic toxicity)
2. Genotoxicity
3. Carcinogenicity
4. Reproductive and developmental toxicity
Non-Applicable Scenarios for TSL:
1. Irritation-related harm
2. Impurities in the "cohort of concern"
3. Unknown compounds
4. Prolonged exposure to devices intended for infants under six months
5. Volatile compounds in inhalable medical devices
EEDmax (Worst Case Estimated Exposure Dose)
EEDmax refers to the maximum exposure dose under specific intended clinical use scenarios.
- For components with short-term contact and available drug release/dissolution kinetics, EEDmax should be calculated based on this information.
- For long-term or persistent contact, or for components without drug release/dissolution kinetics, EEDmax can be calculated based on the total or extractable amount of device components.
Refer to ISO 10993-17:2023 for specific calculation details.
MoS (Margin of Safety)
MoS represents the ratio between the toxicological threshold of concern (TI or TCL) and the estimated exposure dose (EEDmax).
When the MoS value for a substance is greater than 1, the exposure level is considered to pose no significant health risks, and the toxicological risk is deemed acceptable.
JJR Laboratory in China Offers ISO 10993 biocompatibility testing
Feel free to contact us for consultation on iso 10993 testing services!
Email:hello@jjrlab.com
Write your message here and send it to us
 What are the export compliance for electric fans
What are the export compliance for electric fans
 Amazon US Site Electric Fan UL507 Certification
Amazon US Site Electric Fan UL507 Certification
 What Certifications for Electric Fans in the EU &a
What Certifications for Electric Fans in the EU &a
 How to Choose a Test Laboratory for Amazon UL 507?
How to Choose a Test Laboratory for Amazon UL 507?
 Humidifier UL 60335 Test Report for Amazon US
Humidifier UL 60335 Test Report for Amazon US
 Neck-Hanging Fan EU CE Compliance
Neck-Hanging Fan EU CE Compliance
 What is SAR Testing for Absorption Ratio
What is SAR Testing for Absorption Ratio
 Children's Model Toys Amazon CPC Testing Service
Children's Model Toys Amazon CPC Testing Service
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




