
In Vitro Biocompatibility Testing of Biomaterials and Medical Devices
Short-term contact between materials and the body can cause toxicity, irritation, teratogenicity and local inflammation to cells and the whole body; long-term contact may have mutagenic, teratogenic and carcinogenic effects; contact with blood can cause abnormal coagulation function and hemolysis, etc. Therefore, when considering the use of materials in the biomedical field, their biocompatibility is an important indicator that needs to be considered and evaluated.


Biocompatibility test items
Biocompatibility testing
Cytotoxicity assay
Sensitization test
Skin irritation test
Intradermal reaction test
Stimulation test
Service Background
Biocompatibility refers to the ability of a material to elicit an appropriate response in a specific part of the body.
● Short-term contact between the material and the body will cause toxicity, irritation, teratogenicity and local inflammation to cells and the whole body;
● Long-term exposure may be mutagenic, teratogenic and carcinogenic;
● Contact with blood can cause abnormal coagulation function and hemolysis, etc.
Therefore, when considering the use of materials in the biomedical field, their biocompatibility is an important indicator that needs to be considered and evaluated.
Evaluation principles and standards
The evaluation of biocompatibility of materials follows the principles of biosafety and biofunctionality, which requires that biomaterials have very low toxicity and can properly stimulate the corresponding functions of the body in specific applications. The evaluation of biocompatibility mainly refers to the requirements of the International Standards Organization (ISO) 10993 and the national standard GB/T16886, and is carried out through a series of in vitro and in vivo experiments.
Biocompatibility testing items and standards
Test items | Guideline |
Cytotoxicity assay | GB/T 16886.5/ISO 10993-5/GB/T 14233.2/ISO 7405/YY/T 0127.9/YY 0719.7 |
Sensitization test | GB/T 16886.10/iso 10993-10/GB/T 14233.2/GB15979/ YY/T 0879.2 |
Skin irritation test | GB/T 16886.10/ISO 10993-23/GB 15979/YY 0719.7 |
Intradermal reaction test | ISO 10993-10/GB/T 16886.23/GB/T 14233.2 |
Oral irritation test | GB/T 16886.10/ISO 10993-23/ISO 7405/YY/T 0127.13 |
Eye irritation test | GB/T 16886.10/ISO 10993-23/GB 19192/YY 0719.7 |
Vaginal irritation test | GB/T 16886.10/ISO 10993-23 |
Penile stimulation test | GB/T 16886.10/ISO 10993-23 |
Rectal irritation test | GB/T 16886.10/ISO 10993-23 |
Acute systemic toxicity test | GB/T 16886.11/ISO 10993-11/GB/T 14233.2/ISO 7405/YY/T 0127.2/YY/T 0127.5/YY/T 0127.14 |
Pyrogen test | GB/T 16886.11/ISO 10993-11/GB/T 14233.2/Chinese Pharmacopoeia |
Subacute systemic toxicity (14 days, 30 days, 60 days) | GB/T 16886.11/ISO 10993-11/GB/T 14233.2/YY/T 0127.15 |
Subchronic (chronic) systemic toxicity test (90 days, 180 days) | GB/T 16886.11/GB/T 16886.6/ISO 10993-11/ISO 10993-6/GB/T 14233.2/YY/T 0127.15 |
Endotoxin test | GB/T 14233.2/YY/T 0616.1/YY/T 1295/Chinese Pharmacopoeia |
Blood compatibility test | GB/T 16886.4/ISO 10993-4/GB/T 14233.2/ISO 7405/YY/T 0127.1 |
Chromosome aberration test | GB/T 16886.3/ ISO 10993-3/ISO/TR 10993-33/YY/T 0127.16/ YY/T 0870.2/ YY/T 0870.5 |
Micronucleus test | GB/T 16886.3/ ISO 10993-3/ISO/TR 10993-33/YY/T 0127.12/ YY/T 0870.4 |
In vitro mammalian cell micronucleus assay | YY/T 0870.6 |
Gene mutation test | GB/T 16886.3/ ISO 10993-3/ISO/TR 10993-33/YY/T 0127.17/ YY/T 0870.3 |
Implantation test | GB/T 16886.6/ISO 10993-6/ GB/T 14233.2/ISO 7405/ YY/T 0127.8/ YY/T 0127.4 |
Ames test | GB/T 16886.3/ ISO 10993-3/ISO/TR 10993-33/ISO 7405/YY/T 0870.1/YY/T 0127.10 |
Bacterial reverse mutation assay | YY/T 0870.1/YY/T 0127.10 |
Applicable product range
Medical devices, cosmetics, disposable sanitary products, disinfection products, etc.
General sample requirements
Representative samples. For other specific details, please consult JJR online customer service.
Email:hello@jjrlab.com
Write your message here and send it to us
 EMC Pre Compliance Testing
EMC Pre Compliance Testing
 PAHs Testing (Food and Textile)
PAHs Testing (Food and Textile)
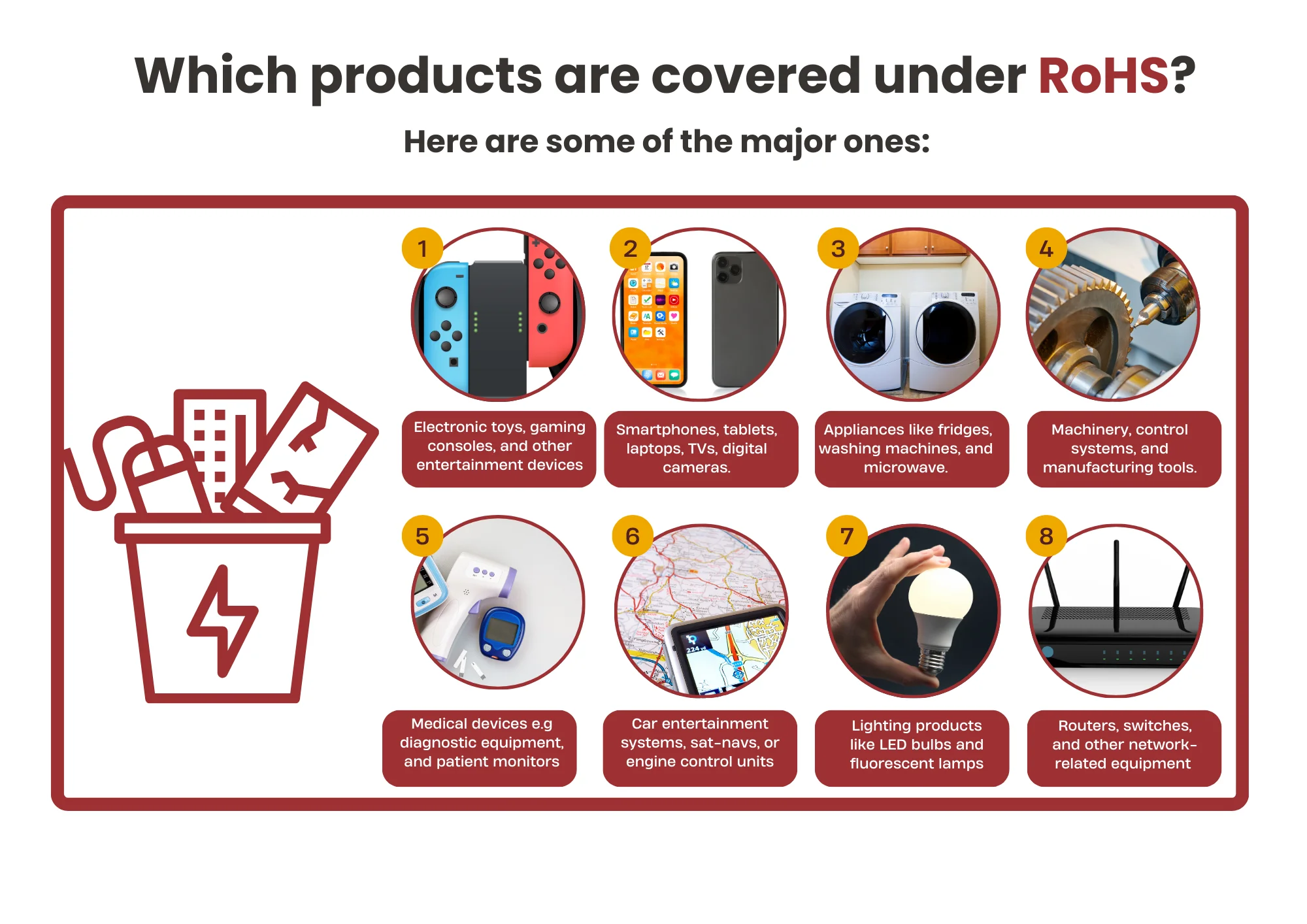 Where to Apply for the EU RoHS Test Report?
Where to Apply for the EU RoHS Test Report?
 Children’s Products and Toy Testing
Children’s Products and Toy Testing
 What is a GB 31701 Test Report?
What is a GB 31701 Test Report?
 UN 38.3 Transportation Test
UN 38.3 Transportation Test
 Toxicological Risk Assessment of Medical Devices
Toxicological Risk Assessment of Medical Devices
 How to get the Vacuum Cleaner UL 1017 Test Report?
How to get the Vacuum Cleaner UL 1017 Test Report?
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




