
IEC 60601-1 Testing Chambers
The safety compliance laboratory for medical products has obtained IAS ISO 17025 accreditation and FDA ASCA recognition from the U.S. government. This enhances confidence in medical device testing and promotes consistency and predictability in the pre-market review process. It encourages the efficient use of FDA resources, supports international harmonization, and improves the efficiency of FDA reviews. This ensures approved products are safe and effective while avoiding unnecessary delays. Consequently, it helps clients smoothly complete company registration, product registration, and FDA Pre-market Notification 510(k) review processes.

Accredited Laboratory Inquiry Link:
https://www.fda.gov/medical-devices/standards-and-conformity-assessment-program/asca-accredited-testing-laboratories
Currently Authorized Medical Products and Common Standards (Partial List):
- IEC 60601-1-11: Home medical products, such as blood pressure monitors, forehead thermometers, air pumps, and massage health devices.
- IEC 60601-1-6: Usability evaluation for all medical products.
- IEC 60601-2-25: Electrocardiographs, used to produce ECG reports for diagnostic purposes.
- IEC 60601-2-27: ECG monitoring devices, used to monitor and record a patient’s cardiac electrical activity and display the data.
- IEC 60601-2-47: Dynamic electrocardiographic devices, capable of continuous recording and analysis of ECGs.
- IEC 60601-2-30: Non-invasive blood pressure monitors.
- ISO 60601-2-56: Temperature measurement devices, including forehead thermometers.
- ISO 60601-2-61: Pulse oximeters.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
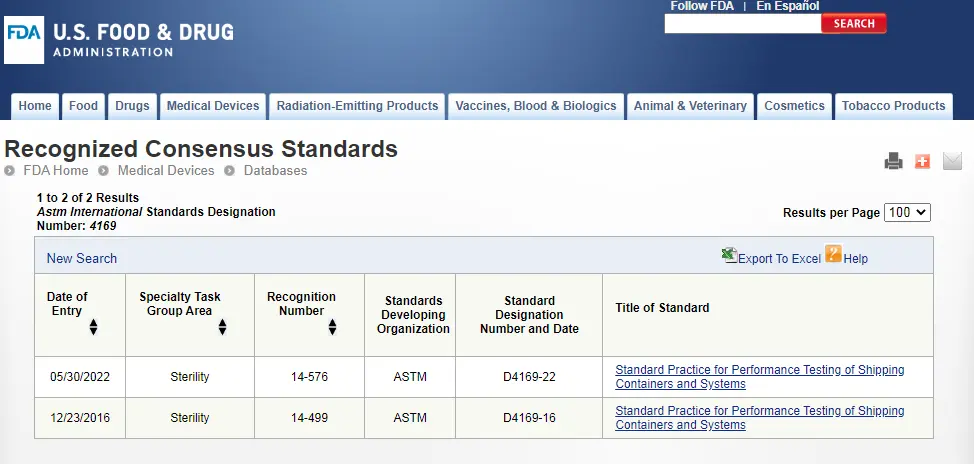 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




