
How to CE Mark a Medical Device?
Medical device CE certification standards
Before launching a medical device in the European Economic Area (EEA), it must obtain the CE mark. The CE mark confirms that the medical device meets certain “essential requirements” of the European General Medical Device Directive (i.e., it is suitable for its intended purpose and is safe). It also indicates that the medical device can be freely sold anywhere in the EEA without further controls.

Even if the medical device is manufactured outside the European Economic Area, CE marking is mandatory and the manufacturer is responsible for obtaining and placing the CE marking on the product. If the product is imported from outside the European Economic Area, the responsibility lies with the importer within the community.
Regulatory Background
There are currently three key European directives governing the requirements for marketing medical devices, and two directives are specific to particular types of devices: active implantable devices (e.g. implantable pacemakers) and in vitro diagnostic medical devices (e.g. assays for determining a patient's sensitivity to a particular drug). This article considers the procedure for obtaining the CE mark under the third directive, which covers all other types of medical devices (from dressings, vascular stents and X-ray machines to spectacles).
Please note that in 2012, the European Commission published proposed legislation to replace these directives. The legislation is expected to be adopted early next year.
Medical device CE certification chooses the conformity assessment route:
To obtain the CE mark for a medical device, the manufacturer must follow one of four conformity assessment procedures, depending on the classification of the medical device. Medical devices can be classified as Class I, Class IIa, Class IIb or Class III.
1) Class I other
2) Class I sterile
3) Class I measurement function
4) Class IIa 2a
5) Class IIb 2b
6) Class III and Class III with medicine
Broadly speaking, devices are classified according to their perceived associated risk, taking into account a number of factors, including how long the device is used continuously, whether it is invasive, and whether it contains any medicinal substances. The higher the perceived associated risk of a device, the stricter the controls imposed on it through the conformity assessment procedure.
Each manufacturer must classify its medical device and choose the appropriate conformity assessment procedure.
Preparation of the evaluation procedure - Technical documentation
For all categories of devices, manufacturers need to provide technical documentation. The requirements for the technical documentation depend on the chosen conformity assessment procedure. As a general rule, the documentation should cover the design, manufacture and intended operation of the product. Ultimately, the manufacturer must provide sufficient information to demonstrate that the device complies with the relevant requirements of the Medical Devices Directive.
The CE certification standards for medical devices are:
(1) EN60601-1 Medical electrical equipment Part 1: General requirements for safety;
(2) EN60601-1-1 Medical electrical equipment Part 1: General requirements for safety and Amendment No. 1;
(3) EN60601-2-11 Medical electrical equipment Part 2: Safety requirements for gamma beam therapy equipment;
(4) EN60601-1-2 Medical electrical equipment Part 1: General requirements for safety Section 1.2 Parallel standard Electromagnetic compatibility - Requirements and tests.
Among them, the standards (1), (2) and (3) are the basis for the Gamma Knife low voltage (LVD) test: The standard (4) is the basis for the Gamma Knife electromagnetic compatibility (EMC) test.
To obtain CE marking, medical device manufacturers will need to:
1. Classify their equipment and choose the appropriate conformity assessment route;
2. Prepare a technical file;
3. Conduct conformity assessment procedures;
4. Obtain a certificate of conformity from a notified body (if applicable);
5. Register with the competent authority (if not already registered).
Email:hello@jjrlab.com
Write your message here and send it to us
 FCC Certification Testing for Smart Lighting Produ
FCC Certification Testing for Smart Lighting Produ
 What is the ETSI EN 303 645 Testing Standard?
What is the ETSI EN 303 645 Testing Standard?
 UL Compliance and ETL Certification for LED Lighti
UL Compliance and ETL Certification for LED Lighti
 What is the IEC 60598 Standard?
What is the IEC 60598 Standard?
 What is the Canada IC Logo?
What is the Canada IC Logo?
 EMC Pre Compliance Testing
EMC Pre Compliance Testing
 PAHs Testing (Food and Textile)
PAHs Testing (Food and Textile)
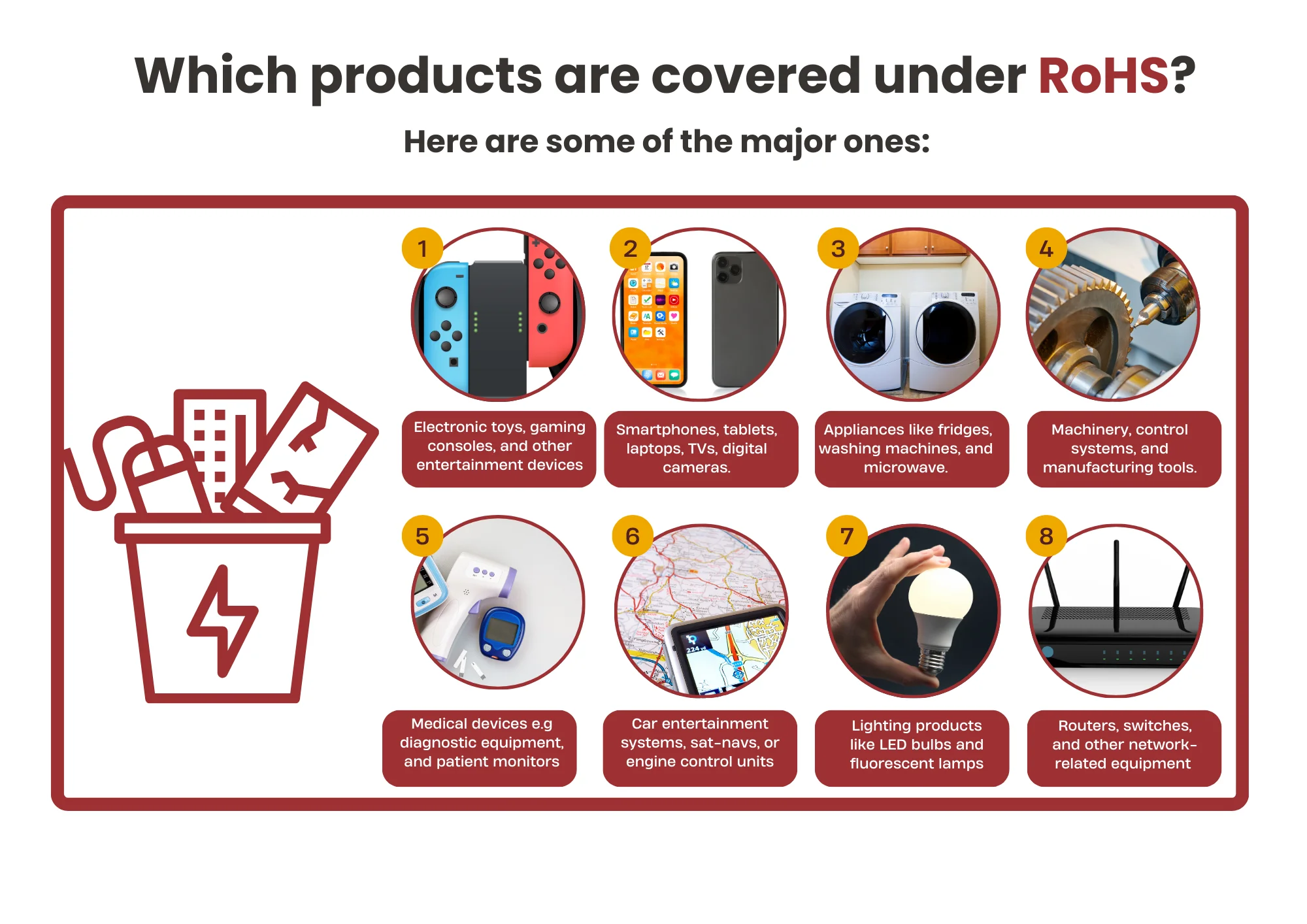 Where to Apply for the EU RoHS Test Report?
Where to Apply for the EU RoHS Test Report?
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




