
How much does ISO 10993 Biocompatibility Testing cost?
Straight to the point, how much does it cost for medical device biocompatibility testing?
In Chinese laboratories, the cost for the standard three biocompatibility tests according to ISO 10993 is $3100 USD; of course, we need your test samples; (EMAIL: CHEN18814186731@GMAIL.COM)

What is biocompatibility?
Biocompatibility refers to the performance of living tissue's reaction to inert materials, generally referring to the compatibility between the material and the host. After biomaterials are implanted into the human body, they have effects on specific biological tissue environments, and biological tissues also have effects on biomaterials. The interaction between the two continues until a balance is reached or the implant is removed. Biocompatibility can be divided into biological reactions and material reactions. Biological reactions include blood reactions, immune reactions, and tissue reactions, while material reactions mainly manifest as changes in the physical and chemical properties of materials. Biocompatibility mainly depends on the nature and application of the material. The properties of the material and the product itself, including shape, size, and surface roughness, as well as toxic low-molecular substances remaining from the polymerization or preparation process of the material, material processing pollution, and degradation products of the material in the body, are all related to its biocompatibility. Materials in short-term contact with the body may cause toxicity, irritation, teratogenicity, and local inflammation to cells and the whole body; long-term contact may have mutagenic, teratogenic, and carcinogenic effects; contact with blood may cause abnormal coagulation function and hemolysis. Therefore, when considering the use of materials in the biomedical field, their biocompatibility is an important indicator that needs to be considered and evaluated.

Biocompatibility applicable products:
1. Products related to active sources:
- Multi-parameter monitors
- Ultrasonic diagnostic equipment
- Infusion pumps and infusion controllers
- Thermometers
- Non-invasive blood pressure monitoring equipment
- Pulse oximetry equipment
- High-frequency electrosurgical equipment
- Nerve and muscle stimulators
- Endoscopes
- Electroencephalographs
- Surgical, cosmetic, diagnostic, and therapeutic laser equipment
- Electrocardiograph equipment
- Clinical chemistry analyzers
- Immunochemistry analyzers
- Fully automated blood analyzers
- Microbiological analyzers
- Fully automated protein analyzers
- Biochemical analyzers
- Hematology analyzers
- Blood gas analyzers
- Chemiluminescence immunoassay analyzers
- Urine sediment analyzers
- Blood coagulation analyzers
- Fully automated hemorheology analyzers
- Fully automated intracellular bacterial culture analyzers
- Microbial identification and bacterial drug sensitivity analyzers
- Nucleic acid purification instruments
- Blood and tissue culture instruments
- Frozen slicers
- Biological tissue dehydrators
- Tissue embedding machines
- Centrifuges
- Mixers
- Stainers
- High-temperature pulsating vacuum sterilizers
- High-temperature steam sterilizers
- Infrared electric sterilizers
- High-temperature disinfection washing machines
- Biological safety cabinets, etc.

2. Products related to passive sources:
Surface-contact devices: electrodes, external prostheses, fixation straps, compression bandages, and various types of monitors, contact lenses, urinary catheters, endoscopic or digestive tract instruments (gastroscopes, colonoscopes, gastroscope), tracheal intubation, bronchoscopes, etc.; dressings or nursing instruments and occlusive dressings for ulcers, burns, granulation tissue, etc.
External access devices: blood transfusion, infusion sets, extension tubes, transfer sets, etc.; laparoscopes, arthroscopes, drainage systems, dental filling materials, skin nails, etc.; intravascular catheters, temporary pacemaker electrodes, dialyzers, dialysis tubing and accessories, vascular adsorbents, immune adsorbents, etc.
Implantable devices: orthopedic nails, artificial joints, bone prostheses, bone cement, and bone internal instruments, pacemakers, implantable drug delivery devices, nerve-muscle sensors and stimulators, artificial tendons, breast implants, artificial larynx, subcutaneous implants, ligatures, etc.; pacemaker electrodes, artificial arteriovenous fistulae, heart valves, artificial blood vessels, intravenous drug delivery tubes, and ventricular assist devices, etc.

Introduction to biocompatibility testing
At present, the standards referred to in biocompatibility testing are ISO10993 and GB/T16886. The content of the two standards is basically the same. The ISO10993 and GB/T16886 standards have clearly stipulated the specific biological evaluation process, and chemical characterization tests need to be conducted before conducting biocompatibility experiments. In vitro diagnostic products do not directly contact the body, so ISO10993 and GB/T16886 standards are not applicable to such products. The safety evaluation of other active and passive medical devices that directly contact the body needs to be tested according to procedures.

What are the biocompatibility testing items?
- In vitro cytotoxicity test
- Mouse typhoid and Salmonella reverse mutation test
- Sensitization test
- Genetic mutation test
- Skin irritation test
- Thrombosis test
- Intradermal irritation test
- Coagulation test
- Acute systemic toxicity test
- Subacute systemic toxicity test
- Platelet adhesion test
- Complement activation test
- Subacute systemic toxicity test
- Chronic systemic toxicity test
- Muscle implantation test
- Hemolysis test
- Thermal source test
- Chromosome aberration test
- Bone implantation test
- Material characterization analysis
- Subcutaneous implantation test
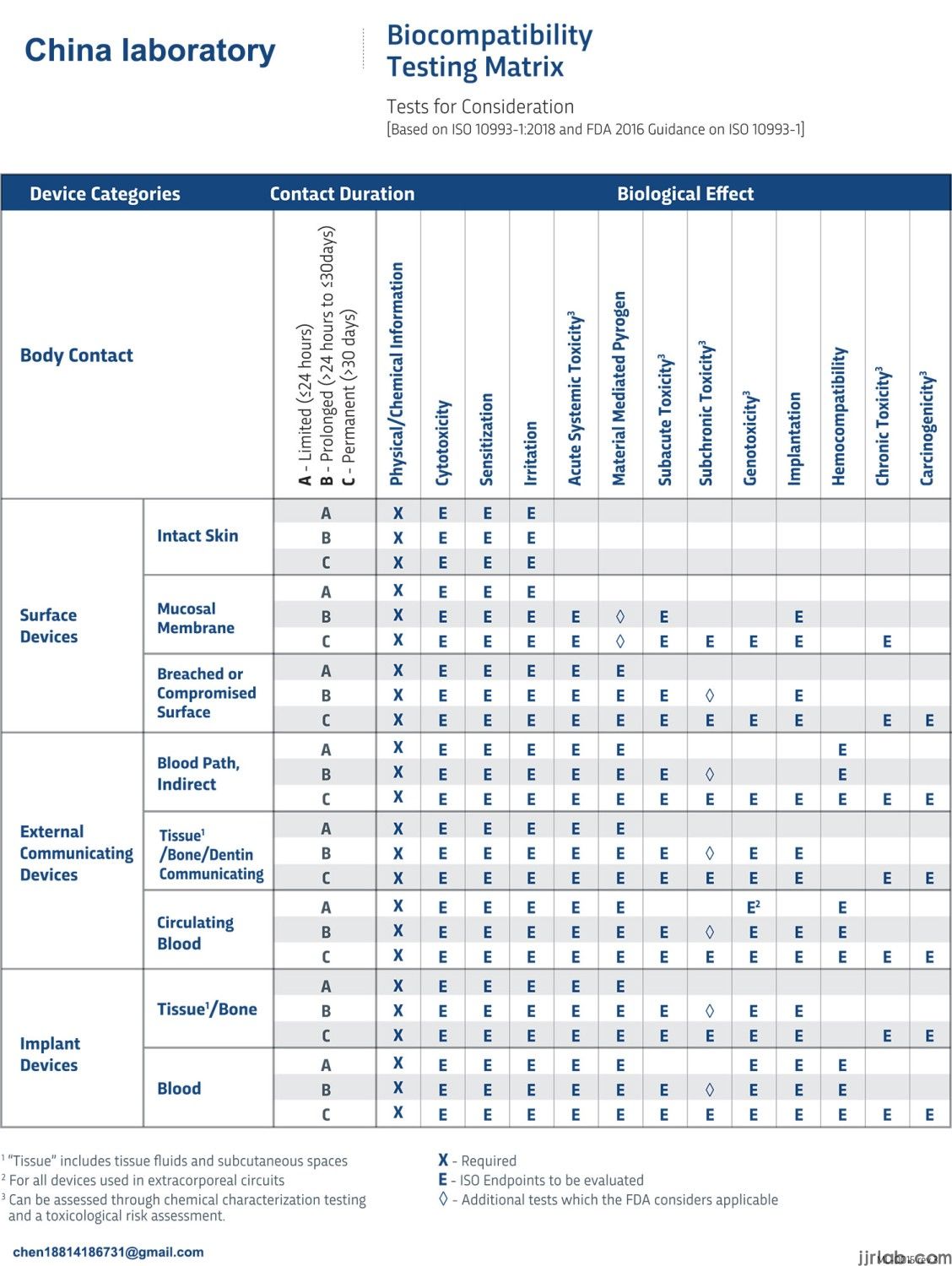
Biocompatibility testing domestic and international standards
1. Cytotoxicity test
- Cytotoxicity test (MTT method) (ISO 10993-5)
- Cytotoxicity test (agar method) (ISO 10993-5 / USP 87)
- Cytotoxicity test (filter membrane method) (ISO 10993-5)
- Cytotoxicity test (direct contact method) (ISO 10993-5 / USP 87)
- Cytotoxicity test (elution method) (USP 87)
2. Skin irritation and sensitization tests
- Sensitization test (maximum dose method / patch method) (iso 10993-10)
- Skin irritation test (ISO 10993-10)
- Intradermal irritation test (ISO 10993-10 / USP 88)
- Oral irritation test (requires histopathology slides) (ISO 10993-10)
- Vaginal irritation test (requires histopathology slides) (ISO 10993-10)
- Penile irritation test (requires histopathology slides) (ISO 10993-10)
- Rectal irritation test (requires histopathology slides) (ISO 10993-10)
- Eye irritation test (ISO 10993-10)
3. Systemic toxicity test
- Acute systemic toxicity test (ISO 10993-11 / USP 88)
- Subacute systemic toxicity test (ISO 10993-11)
- Subchronic systemic toxicity test (ISO 10993-11)
- Thermal source test (ISO 10993-11)
4. Post-implantation local reaction test
- Subcutaneous implantation test (ISO 10993-6)
- Muscle implantation test (ISO 10993-6)
- Bone implantation test (ISO 10993-6)
5. Blood compatibility test
- Hemolysis test (ISO 10993-4 / GB16886.4)
- Hemolysis test (ASTM F756)
- Coagulation test (ISO 10993-4 / GB16886.4)
- Platelet count test
- Complement test (ISO 10993-4 / GB16886.4)
- Thrombosis test (in vivo, in vitro) (ISO 10993-4 / GB16886.4)
6. Genotoxicity / Genetic toxicity test (ISO 10993-3)
- Bacterial recovery test
- Mouse lymphoma test
- Chromosome aberration test
- Micronucleus test (mouse)
If you need further information or have other questions, please feel free to contact us.Email:chen18814186731@gmail.com
Email:hello@jjrlab.com
Write your message here and send it to us
 FCC Certification Fees for Handheld Fans
FCC Certification Fees for Handheld Fans
 FCC Certification Testing for Smart Lighting Produ
FCC Certification Testing for Smart Lighting Produ
 What is the ETSI EN 303 645 Testing Standard?
What is the ETSI EN 303 645 Testing Standard?
 UL Compliance and ETL Certification for LED Lighti
UL Compliance and ETL Certification for LED Lighti
 What is the IEC 60598 Standard?
What is the IEC 60598 Standard?
 What is the Canada IC Logo?
What is the Canada IC Logo?
 EMC Pre Compliance Testing
EMC Pre Compliance Testing
 PAHs Testing (Food and Textile)
PAHs Testing (Food and Textile)
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




