
How Long is the REACH Testing Process?
What is the REACH Testing Project?
According to the REACH regulation (1907/2006/EC), manufacturers and importers must notify the European Chemicals Agency (ECHA) if either of the following conditions is met:
1. The concentration of Substances of Very High Concern (SVHC) in the product exceeds 0.1%.
2. The total quantity of SVHC in the manufacturer’s or importer’s products exceeds 1 ton per year.
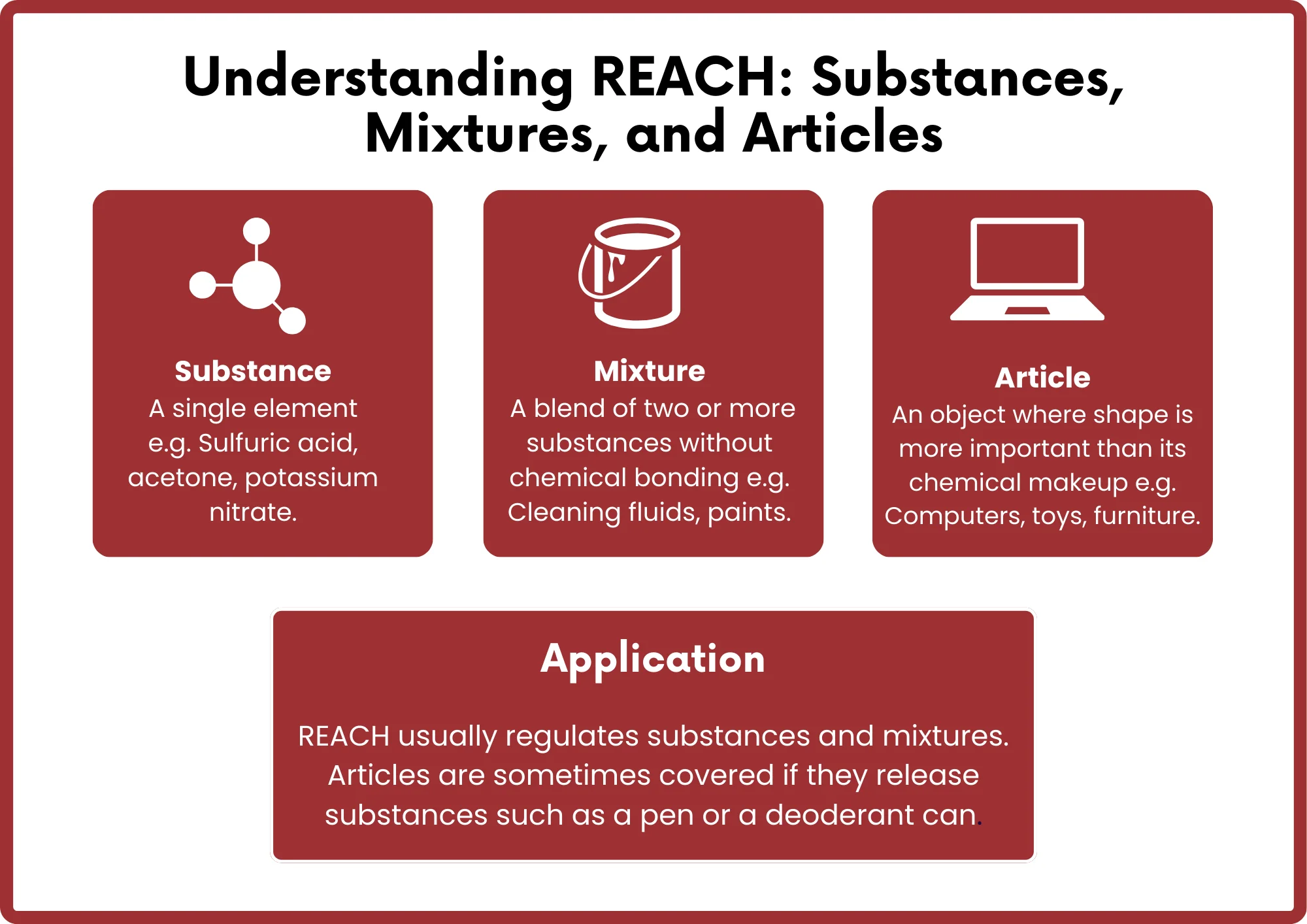
What is the REACH Certification Process?
1. Certification Consultation– Provide relevant product information.
2. Quotation– Determine testing fees and time.
3. Application Form– Fill out the application, sign the contract, and send the sample.
4. Testing– The sample is tested by the Chinese JJR laboratory according to REACH standards.
5. Report Issuance– A REACH report is issued upon passing the test.
6. Delivery Service– The REACH certification report and invoice are sent to the applicant.
How Much Does a REACH Certification Cost?
The cost depends on the material of the product.
- Metal materialsrequire testing for 59 items.
- Non-metal materialsrequire testing for 191 items, making them more expensive.
There are two testing methods: individual testing and mixed testing.
- Metal materials: REACH certification costs between $560 - $700.
- Non-metal materials: REACH certification costs between $680 - $880.
How Long Does REACH Certification Take?
The REACH certification process typically takes around one week. For complex products, the testing period may be longer.
How Long is the REACH Certification Valid?
REACH certification does not have an expiration date. If the certified product remains unchanged, the REACH report can be used indefinitely.
What is the Purpose of REACH Testing?
1. Submission of hazardous chemical information.
2. Management of hazardous chemicals.
3. Registration of the chemical substance for all intended uses.
4. Encouragement of industrial development.
5. Promotion of substances with lower health risks.
6. Promotion of substances with lower environmental risks.
7. Support for EU authorities in taking quicker action against chemical risks.
8. Enhancing the competitiveness of EU chemical enterprises to boost economic growth.
9. Development of methods to manage hazardous substances.
10. Ensuring the free circulation of substances within the EU internal market.
Email:hello@jjrlab.com
Write your message here and send it to us
 Toothbrush FDA Certification Testing
Toothbrush FDA Certification Testing
 Snoring Device FDA 510k Standard Testing
Snoring Device FDA 510k Standard Testing
 Single Use Intravenous Catheter Certification Test
Single Use Intravenous Catheter Certification Test
 Silicone Material Product Compliance Certification
Silicone Material Product Compliance Certification
 What to Do If Cytotoxicity Test Results Are Positi
What to Do If Cytotoxicity Test Results Are Positi
 ISO 10993:5 Cytotoxicity Testing Methods
ISO 10993:5 Cytotoxicity Testing Methods
 FDA ISO 10993-1 Biocompatibility Evaluation Guidel
FDA ISO 10993-1 Biocompatibility Evaluation Guidel
 In Vitro Cytotoxicity Testing for Medical Devices
In Vitro Cytotoxicity Testing for Medical Devices
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




