
EMC Radio Frequency Electromagnetic Field Immunity Test
In the widespread application of modern electronic devices and systems, the conversion and transmission of electromagnetic energy have become indispensable. However, the presence of this electromagnetic energy often causes significant interference with communication systems, control systems, and computer systems, particULarly affecting sensitive components such as integrated circuits. Therefore, the Radiated Susceptibility Test (RS Test), as a fundamental part of Electromagnetic Compatibility (EMC) testing, is of paramount importance.

Radio Frequency Electromagnetic Field Immunity Test
The Radio Frequency Electromagnetic Field Immunity Test is a critical emc test used to evaluate the ability of a device to operate in a strong electromagnetic field environment. This test verifies whether a device can maintain normal functionality when exposed to radio frequency (RF) electromagnetic fields of specified frequency and intensity. It is widely applied in consumer electronics, medical devices, industrial equipment, and other fields.
Test Setup
1. Test Environment
- Shielded Room/Anechoic Chamber: The test is typically conducted in an anechoic chamber to avoid external interference.
- Equipment Placement: The Equipment Under Test (EUT) should be placed on a non-conductive table, at a height of 0.8 meters or a specified level above the ground. The antenna is usually positioned 1 meter from the EUT.
2. Calibration Steps
- Field Strength Calibration: Before placing the EUT, an electromagnetic field probe is used to calibrate the field strength in the test area, ensuring field uniformity within ±1.5 dB.
- Setting Frequency Range and Steps: According to standard requirements, the test frequency range is set (typically from 80 MHz to 6 GHz), with a step size usually set to 1%.
Test Objective
The purpose of the Radio Frequency Electromagnetic Field Immunity Test is to evaluate the EUT’s resistance to RF electromagnetic fields and to determine whether it can function normally under a pREDetermined electromagnetic environment.
Test Standards
Commonly used test standards include:
- iec 61000-4-3: Electromagnetic Compatibility (EMC) – Part 4-3: Testing and Measurement Techniques – Radiated, Radio-Frequency, Electromagnetic Field Immunity Test.
- en 61000-4-3: The European standard equivalent to IEC 61000-4-3.
Test Equipment
- Signal Generator: Generates RF signals within the required frequency range.
- Power Amplifier: Amplifies the RF signals from the signal generator to achieve the required field strength.
- Antenna: Common types include log-periodic antennas and biconical antennas, used to radiate RF electromagnetic fields.
- Electromagnetic Field Probe: Monitors and calibrates the electromagnetic field strength in the test area.
- Shielded Room or Anechoic Chamber: Isolates external electromagnetic interference, providing a stable test environment.
Test Results Analysis
The analysis of radiated immunity test results is crucial for assessing a device's resistance to electromagnetic interference. By examining test results, one can evaluate the device’s performance in an electromagnetic field, identify potential issues and weaknesses, and propose corresponding improvement measures.
For example, if the test reveals significant performance degradation at a specific frequency, an analysis of the radiation characteristics at that frequency can help optimize the device’s electromagnetic shielding design or signal transmission pathways to reduce electromagnetic interference.
Additionally, test results can be compared with industry standards or product specifications to determine whether the device meets the required immunity levels. If the test results do not meet the standards, further investigation is needed to identify the cause and implement necessary improvements until compliance is achieved.
Email:hello@jjrlab.com
Write your message here and send it to us
 What is Protection Class EN 60529?
What is Protection Class EN 60529?
 IP69 Certified Protection
IP69 Certified Protection
 California Energy Commission Testing Lab
California Energy Commission Testing Lab
 What Does the Canadian IC Mark Mean?
What Does the Canadian IC Mark Mean?
 How Much is the Canada IC ID Certification cost?
How Much is the Canada IC ID Certification cost?
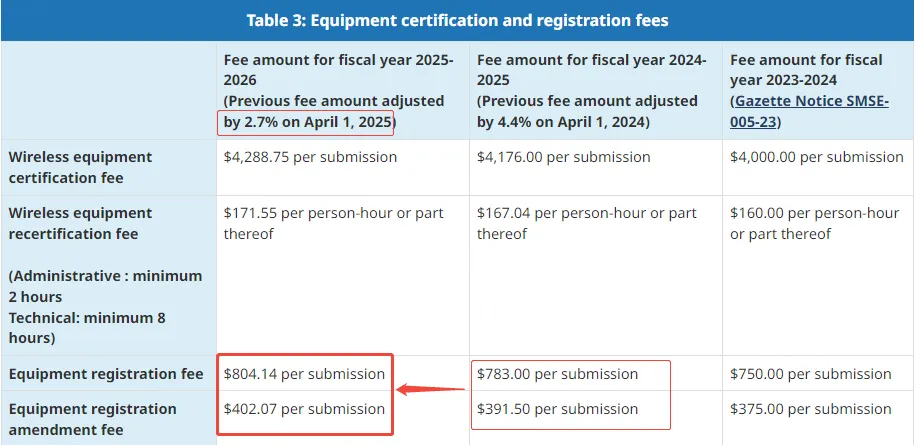 How Much is the Canada IC ID Certification Fee?
How Much is the Canada IC ID Certification Fee?
 How Much is the UL 982 Test Report csost?
How Much is the UL 982 Test Report csost?
 ISO/IEC 17025 Accredited Test Laboratory
ISO/IEC 17025 Accredited Test Laboratory
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




