
Electrocardiograph Certification Testing Laboratory
Electrocardiogram (ECG):
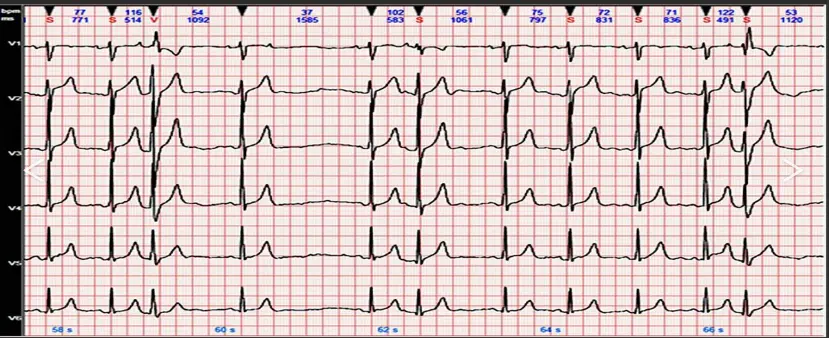
The heart is primarily composed of numerous myocardial cells. The sinoatrial node in the heart wall periodically emits electrical signals, which are transmitted to various parts of the heart through structures such as the internodal pathways and Purkinje fibers. This conduction causes excitation and accompanying potential changes throughout the heart, leading to regular heart contractions that maintain and coordinate the normal rhythm of the heart. During this process, the potential changes in different parts of the heart spread to the body surface. By placing chest electrodes or limb electrodes on the body surface, these potentials can be picked up and recorded as a time-varying curve, known as an electrocardiogram.
Electrocardiograph:
An instrument that records the time-varying ECG waveform at various points on the body surface is called an electrocardiograph.
Who needs an electrocardiogram?
1. Individuals experiencing discomfort in the precordial area, such as chest tightness, palpitations, shortness of breath, chest pain, or transient vision loss, should undergo an ECG examination to rule out myocardial ischemia, premature beats, tachycardia, conduction block, and other cardiac conditions.
2. Patients with common cardiovascular diseases, such as coronary heart disease, primary hypertension, myocardial infarction, and stroke. ECGs are crucial for monitoring disease trends, identifying early preventive measures, and objectively evaluating the effects of certain cardiovascular medications and treatments. Such individuals should have regular ECGs for clinical reference.
3. Patients with diarrhea or abdominal pain, regardless of age, should also undergo an ECG examination. Severe diarrhea can cause electrolyte imbalances, and an ECG can assist in diagnosing potassium levels. Abdominal pain may also indicate myocardial infarction.
4. Discharged patients, especially those with abnormal ECGs upon admission, should have an ECG upon discharge to compare with the admission ECG, aiding doctors in formulating discharge instructions.
Importance of ECG Testing
- Understanding Basic Conditions: The heart is essentially the body's engine, and any disease can potentially harm it. Doctors can use ECGs to assess whether the patient's heart rate and rhythm are normal, providing a basic evaluation of heart function.
- Assisting in Diagnosing Coronary Heart Disease: While ECG alone cannot completely diagnose coronary heart disease, it can aid in diagnosis. During an acute myocardial infarction, an ECG can quickly identify and localize the infarction, providing essential information for clinical treatment.
- Assessing Myocardial Damage: Some heart diseases also involve myocardial damage. ECGs can preliminarily determine the presence of such damage.
- Diagnosing Arrhythmias: ECG is the only diagnostic method for arrhythmias. Before prescribing medication, doctors need to know if the patient has a history of arrhythmias, as some drugs can induce arrhythmias.
- Evaluating Treatment Effectiveness: For conditions like acute myocardial infarction, thrombolytic therapy can be evaluated by comparing ECGs before and after treatment. During patient resuscitation, an ECG can promptly reflect the heart's recovery status.
- Assisting in Diagnosing Other Diseases: ECGs can help diagnose diseases caused by electrolyte imbalances such as hypokalemia and hyperkalemia, as well as myocardial hypertrophy due to various causes.
Conclusion: Although small, the ECG plays a significant role. We hope this article has helped you better understand the importance of ECGs.
China JJR Laboratory Certification Testing Solutions
EMC:
YY 9706.102 | IEC 60601-1-2 (EMC)
Safety:
GB 9706.1 | IEC 60601-1
YY/T 9706.106 | IEC 60601-1-6 (Usability)
GB/T 14710 (Medical Electrical Equipment Environmental Requirements)
YY 1057 (Medical Foot Switch)
GB 9706.225 | IEC 60601-2-25 (Electrocardiograph)
YY 9706.247 | IEC 60601-2-47 | ANSI/AAMI EC38 (Ambulatory ECG System)
YY 0782 | IEC 60601-2-51 (Single and Multi-channel ECG Recorders and Analyzers)
YY 1139 | ANSI/AAMI EC11 (ECG Diagnostic Devices)
YY/T 0196 (Single-use ECG Electrodes)
YY 0828 | ANSI/AAMI EC53 (ECG Monitor Cables and Leadwires)
Biocompatibility:
GB/T 16886.5 / ISO 10993-5 (Cytotoxicity)
GB/T 16886.10 / iso 10993-10 (Skin Sensitization)
GB/T 16886.10 / ISO 10993-23 (Skin Irritation)
Reliability Testing & Failure Analysis:
IEC 60068, IEC 60529; IEC 60598, EIA-364, MIL-STD-202, ISO 4892, ISO 1431, ASTM G 154, ASTM G155, ASTM D4728, etc. (Reliability Testing)
IPC/ECAJ-STD, IPCTM 650-2, ISO 16232-10, etc. (Failure Analysis)
Packaging and Transportation:
GB/T 4857 / ISTA Series / ASTM D 4169
Microbiological Testing:
Total Anaerobic Bacteria: GB/T 19973.1 / ISO 11737-1
Total Non-selective Aerobic Bacteria: ISO 11737-1
Microorganisms: GB/T 19973.1 / Chinese Pharmacopoeia
Antibacterial Effectiveness: Chinese Pharmacopoeia
Sterility Test: Chinese Pharmacopoeia / GB/T 19973.2 / ISO 11737-2
Total Yeast Count: GB/T 19973.1 / ISO 11737-1
Microbial Limits: ISO 11737
Note: For single-use sterile ECG electrodes, consider the above testing items.
Shelf Life Validation:
Accelerated Aging Test: YY/T 0681.1 / ASTM F1980-16
Seal Peel Test (Seal Strength): YY/T 0681.2 / ASTM F88/F88M
Burst and Creep Test: YY/T 0681.3; ASTM F1140/F1140M
Dye Penetration Test (Seal Leakage): YY/T 0681.4 / ASTM F1929-15
Vacuum Leakage Test: YY/T 0681.5
Seal Burst Test: YY/T 0681.9
Visual Inspection: YY/T 0681.11 / ASTM F1886/F1886M
Microbial Barrier Testing: YY/T 0681.14 / ISO 11607-1 / GB/T 19633.1
Note: For single-use sterile ECG electrodes, consider the above testing items.
Sterilization Validation:
Radiation Sterilization: GB 18280 Series / ISO 11137 Series
Ethylene Oxide Sterilization: GB 18279 Series / ISO 11135 Series
Ethylene Oxide Residuals (EO/ECH): GB/T 16886.7 / ISO 10993-7 / GB/T 14233.1
Cleaning and Disinfection Sterilization Validation: WS 310.1 / WS 310.2 / WS 310.3 / AAMI TIR12 / AAMI TIR30 / FDA Guidance, etc.
Note: For single-use sterile ECG electrodes, consider the above testing items.
Software Evaluation and Cybersecurity Testing:
GB/T 25000.51 "Systems and Software Engineering Systems and Software Quality Requirements and Evaluation (SQuaRE) Part 51: Quality Requirements and Testing for Ready-to-Use Software Products (RUSP)"
GB/T 25000.10 "Systems and Software Engineering Systems and Software Quality Requirements and Evaluation (SQuaRE) Part 10: System and Software Quality Models"
YY/T 1843-2022 "Basic Requirements for Cybersecurity of Medical Electrical Systems and Medical Device Software"
IEC/TR80001-2-2 Application of Risk Management for IT-networks Incorporating Medical Devices — Part 2-2: Guidance for the Communication of Medical Device Security Needs, Risks, and Controls
Note: The above testing items are not exhaustive; only common standards are listed.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
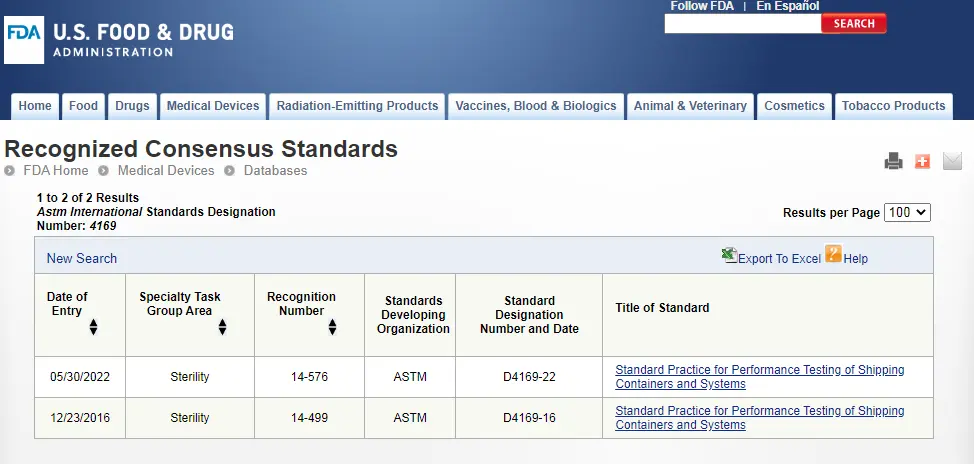 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
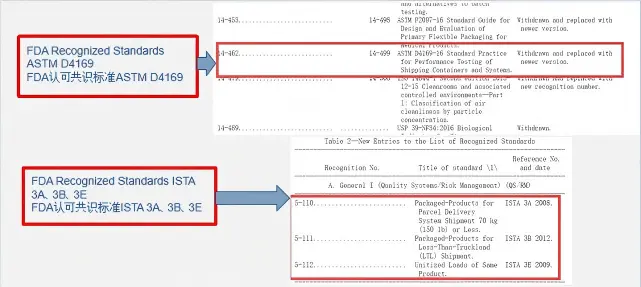 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




