
Cytotoxicity Testing for Cosmetics
Before assessing the efficacy of a new cosmetic or cosmetic ingredient through in vitro testing, it's imperative to understand its safety profile regarding cellular interactions.
What is cytotoxicity testing, and How is it Conducted?
This article will provide a comprehensive explanation.
What is In Vitro Cytotoxicity Testing?
In vitro cytotoxicity testing involves utilizing cell culture techniques to simulate growth environments ex vivo, assessing the dissolution of cells, inhibition of cell growth, or other toxic effects occurring upon exposure to tissues or cells by medical devices, materials, or drugs.
Characteristics of In Vitro Cytotoxicity Testing
Cytotoxicity testing is versatile, applicable to various medical devices and materials evaluations. It can determine whether samples cause cell damage, inhibit cell growth, affect cell proliferation, or colony formation.
Importance of In Vitro Cytotoxicity Testing
Cytotoxicity testing forms the foundational data for evaluating cosmetic efficacy and selecting safe concentrations for adding active ingredients. While certain chemicals may exhibit no apparent toxicity to cells within a specific concentration range, surpassing the safe concentration threshold can lead to cellular damage and decreased cell viability.
Prior to cosmetic efficacy evaluations, specific to different requirements, the cytotoxicity of test substances is assessed. Indirect indicators such as cell count and observation of cell growth status are used to reflect the cytotoxicity of the substance, thereby determining safe concentrations of test substances.
Cytotoxicity Evaluation Methods
1. Qualitative Assessment
Microscopic examination of cells after exposure to test samples for a certain period, observing changes such as alterations in cell morphology, vacuole formation, detachment, cell lysis, and cell membrane integrity.
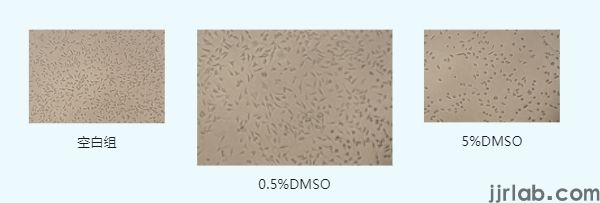
For example, when 5% and 0.5% concentrations of dimethyl sulfoxide (DMSO) act on mouse fibroblast L929 cells for 24 hours, compared to the control group, some cells in the 0.5% DMSO group become transparent, round, and shrink, losing their original morphology; in the 5% DMSO group, most cells exhibit abnormal round shrinkage.
2. Quantitative Assessment
(1) MTT Assay
The MTT assay evaluates cell viability and growth by measuring metabolic activity. It relies on the reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to insoluble blue-purple formazan crystals by succinate dehydrogenase in active mitochondria, which are then dissolved by DMSO. The absorbance at 490 nm reflects the amount of formazan generated, indirectly indicating the number of viable cells. By measuring formazan production, cell viability is calculated, thereby selecting safe concentrations of test samples.
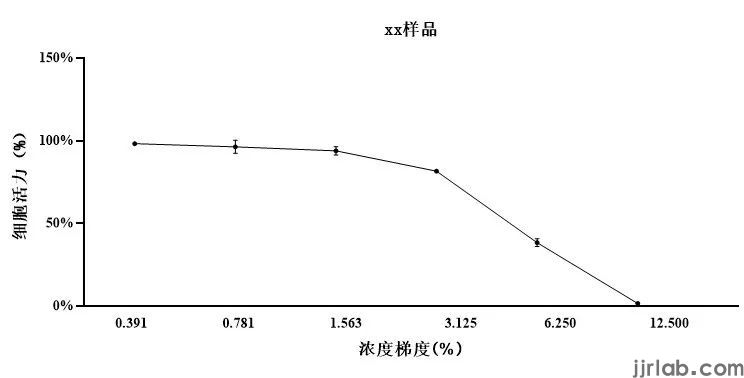
Based on MTT results, sample concentrations with cell viability greater than 90% are chosen.
(2) CCK-8 Assay
The CCK-8 assay detects cell viability and growth through a redox reaction. WST-8 in the CCK-8 reagent reacts with dehydrogenases in active mitochondria to produce water-soluble orange formazan. The absorbance at 450 nm reflects the amount of formazan generated, correlating with cell proliferation and inversely with cytotoxicity.
The CCK-8 assay offers higher sensitivity, better reproducibility, lower cytotoxicity, and suitability for high-throughput drug screening compared to the MTT assay, albeit at a higher cost.
(3) ATP Luminescence Assay
The ATP luminescence assay analyzes cell viability and proliferation by measuring intracellular ATP levels. Endogenous ATP serves as the primary energy source for viable cells, rapidly hydrolyzed upon cell death. Exogenously added firefly luciferase/luciferin reacts with intracellular ATP, converting chemical energy into luminescence, which is detected to quantify ATP content.
If your product requires cytotoxicity testing, please contact us at Email: hello@jjrlab.com.
Email:hello@jjrlab.com
Write your message here and send it to us
 Amazon UL Standard Test Report
Amazon UL Standard Test Report
 When Can FCC ID Modifications Be Filed?
When Can FCC ID Modifications Be Filed?
 LoRa Certification Testing Laboratory
LoRa Certification Testing Laboratory
 Blood Pressure Monitor Certification Testing Servi
Blood Pressure Monitor Certification Testing Servi
 ECG Device Certification Testing
ECG Device Certification Testing
 Pulse Oximeter Certification and Testing Standards
Pulse Oximeter Certification and Testing Standards
 IVD Medical Device GB 4793:2024 Test Report
IVD Medical Device GB 4793:2024 Test Report
 IECEE CBTL Testing Laboratory for IVD Medical Devi
IECEE CBTL Testing Laboratory for IVD Medical Devi
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




