
CCK Cell Toxicity Assay Method
The Cell Counting Kit-8 (CCK-8) reagent is used for convenient and accurate analysis of cell proliferation and toxicity.
Basic Principle:
This reagent contains WST-8 [Chemical name: 2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt], which is reduced by cellular dehydrogenases to a highly water-soluble yellow-colored formazan dye in the presence of the electron carrier 1-Methoxy PMS (1-Methoxy PMS). The amount of formazan produced is proportional to the number of viable cells. Therefore, this property can be used directly for cell proliferation and toxicity analysis.

Applications:
Drug screening, cell proliferation assays, cell toxicity assays, tumor drug sensitivity tests.
Experimental Procedure
1. Cell Proliferation Analysis
1. Prepare cell suspension: Count cells.
2. Inoculate into 96-well plate: Add approximately 100μL of cell suspension per well based on the appropriate seeding density, with three replicates for each sample.
3. Incubate at 37°C: Cells may require incubation for approximately 2~4 hours after seeding to adhere to the well. This step can be skipped if no adhesion is needed.
4. Add 10μL CCK8: Since the amount of CCK8 added per well is relatively small, there may be errors due to the reagent adhering to the well walls. It is recommended to gently tap the culture plate after adding the reagent to ensure thorough mixing. Alternatively, prepare culture media containing 10% CCK8 and add it as a replacement.
5. Incubate for 1-4 hours: The amount of formazan formed varies with cell type. If the color development is insufficient, continue incubation to determine the optimal conditions. Particularly, blood cells may require longer color development times (5-6 hours).
6. Measure absorbance at 450nm: It is recommended to use dual-wavelength measurement, with detection wavelength at 450~490nm and reference wavelength at 600~650nm.
2. Cell Toxicity Analysis
1. Prepare cell suspension: Count cells.
2. Inoculate into 96-well plate: Add approximately 100μL of cell suspension per well based on the appropriate seeding density, with three replicates for each sample.
3. Incubate at 37°C: Cells may require incubation for approximately 2~4 hours after seeding to adhere to the well. This step can be skipped if no adhesion is needed.
4. Add different concentrations of toxic substances.
5. Incubate at 37°C: Incubation time with toxic substances depends on the properties of the substances and the sensitivity of the cells, generally at least one generation time or longer according to the cell cycle.
6. Add 10μL CCK8: Since the amount of CCK8 added per well is relatively small, there may be errors due to the reagent adhering to the well walls. It is recommended to gently tap the culture plate after adding the reagent to ensure thorough mixing.
7. Incubate for 1-4 hours: The amount of formazan formed varies with cell type. If the color development is insufficient, continue incubation to determine the optimal conditions. Particularly, blood cells may require longer color development times (5-6 hours).
8. Measure absorbance at 450nm: It is recommended to use dual-wavelength measurement, with detection wavelength at 450~490nm and reference wavelength at 600~650nm.
Notes and Experience:
1. When using a standard 96-well plate, the minimum seeding volume for adherent cells is at least 1,000 cells/well (100 μL medium). The sensitivity for detecting leukocytes is relatively low, so a seeding volume of at least 2,500 cells/well (100 μL medium) is recommended.
2. Phenol red and serum do not interfere with the CCK8 method, and background absorbance in blank wells can be subtracted.
3. CCK8 can detect Escherichia coli but not yeast cells. Bacterial contamination should be avoided during each measurement in cell proliferation experiments to avoid affecting the results.
4. CCK-8 can be stored for at least 6 months at 0~5°C and for 1 year at -20°C in the dark.
5. When incubating in a CO2 incubator, the outer wells of the culture plate are more prone to drying and evaporation, leading to volume inaccuracies and increased errors. Generally, only the outermost wells are filled with culture medium and not used for measurements.
6. Adding CCK8 to culture medium, incubating for a certain period, and measuring absorbance at 450 nm serve as a blank control. When conducting drug experiments, drug absorption should also be considered. CCK8 can be added to culture medium containing drugs, incubated for a certain period, and absorbance at 450 nm measured as a blank control.
7. Metals affect CCK-8 color development: Lead chloride, iron chloride, and copper sulfate at final concentrations of 1 mM inhibit 5%, 15%, and 90% of color development, respectively, leading to decreased sensitivity. At a final concentration of 10 mM, 100% inhibition occurs.
8. Staining is more challenging for suspension cells, requiring an increase in cell number and prolonged incubation time.
Medical Device Cytotoxicity Testing Email:hello@jjrlab.com
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
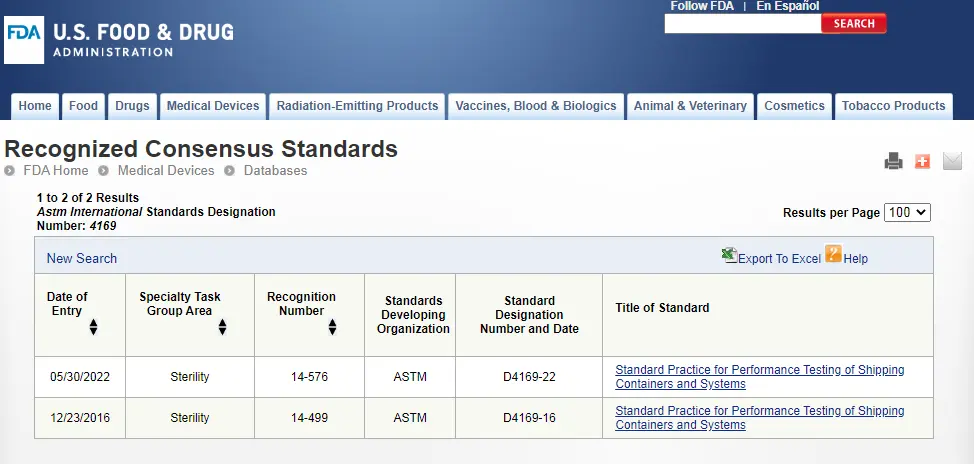 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
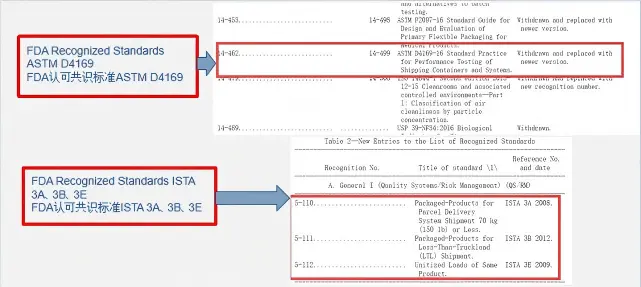
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




