
ASTM D4169 Packaging Simulation Transportation Test
The transportation process of sterile medical device packaging, from manufacturers, sterilization processors, distributors, to the final end-user, may impact the outer carton, primary packaging, and product performance of the final sterilized medical devices. To ensure that sterile medical device packaging and products are not damaged during transportation, we can simulate the transportation process to verify whether the performance of the delivered device still meets the expected performance requirements at the time of manufacture. Both domestic and international standards and regulatory requirements have been released for packaging transportation testing.
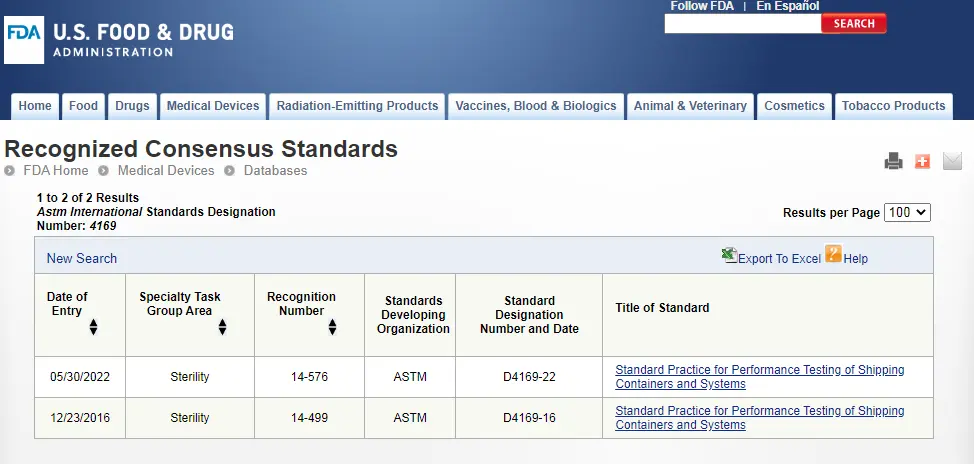
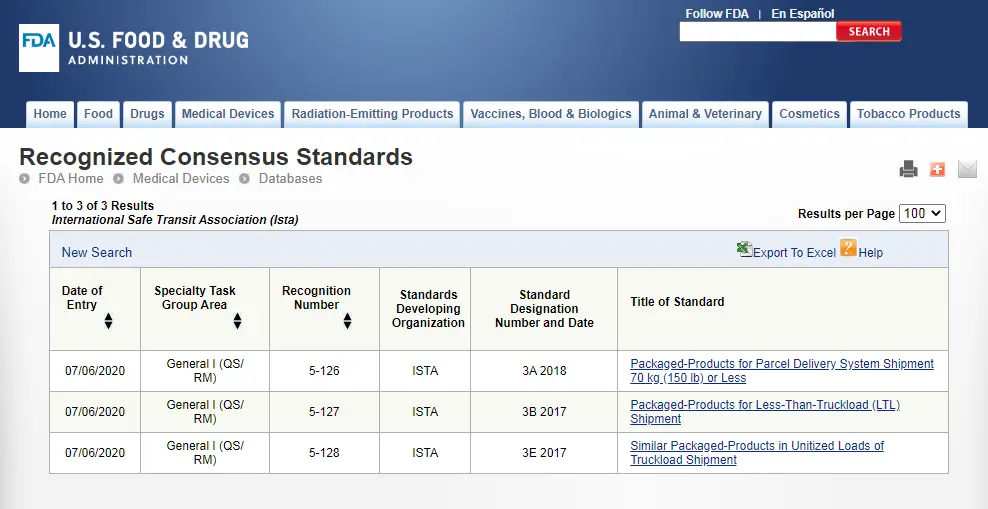
astm d4169-2022 DC13 is an internationally recognized standard for sterile medical device packaging simulation transportation testing and is the FDA's recommended standard. The following introduces the testing process of ASTM D4169-2022 DC13, brought to you by the JJR Laboratory in China.
Test Introduction
Transport Packaging
Transport packaging is designed to minimize the damage caused to products during transportation, ensure the safety of products, facilitate storage, handling, and delivery, and accelerate inspection at handover points. Packaging used primarily for transportation and storage purposes is referred to as transport packaging. Also known as outer packaging, its primary function is to protect goods, prevent damage or discrepancies during storage and transport, minimize the impact of external conditions during transport, and facilitate inspection, counting, and distribution.
Packaging Simulation Transportation Test
This test simulates potential hazards encountered during the transportation process and helps assess the risks at various stages of transport, thereby reducing costs.
Test Objectives:
- Evaluate the quality and effectiveness of the packaging. That is, under certain transportation conditions, test whether the packaging provides adequate protection.
- Examine potential damages caused by the packaging and study the causes, corrective measures, and preventive measures.
- Compare the advantages and disadvantages of different packaging.
Standard Selection
FDA-Approved Packaging Transportation Testing Standards
- ASTM D4169 and the ISTA series, among other standards.
GB/T 19633.1-2015 Final Sterilized Medical Device Packaging Transportation Verification Recommended Standards
Performance tests
- GB/T 4857.17-1992 Packaging, Transport Packaging, Performance Testing
- ASTM D4169:2001 Performance Testing of Transport Containers and Systems
- ISTA 1, 2, and 3 Series International Safe Transport Association Pre-shipment Test Procedures
- YY/T 0698.8-2009 Final Sterilized Medical Device Packaging Materials
YY/T 0681.15:2019 Sterile Medical Device Packaging Transportation Testing Standards
Test Method
ASTM D4169 is currently the widely recognized and comprehensive testing system for evaluating packaging transportation performance.
ASTM D4169 specifies 18 different distribution cycles (DC) and corresponding test procedures based on different types of transportation. Each test procedure consists of several test items (e.g., drop, vibration, pressure tests) and the specific testing conditions are chosen based on the established reliability level.
ASTM D4169 provides a test plan for conducting simulation transportation tests on transport containers and systems, outlining the potential risks during transportation and providing corresponding test items to simulate or replicate these risks, such as drops, impacts, and more.
Common Hazard Factors
- Process A: Hazard factor is manual and mechanical handling, tested for drops, impacts, and stability.
- Process B: Hazard factor is warehouse stacking, tested for pressure.
- Process C: Hazard factor is transport stacking, tested for pressure.
- Process D: Hazard factor is stack vibration, tested for vibration.
- Process E: Hazard factor is transport vibration, tested for vibration.
- Process F: Hazard factor is loose cargo vibration, tested for cyclic vibration.
- Process G: Hazard factor is rail track transitions, tested for horizontal shock.
- Process H: Hazard factor is environmental hazards, tested for cyclical exposure.
- Process I: Hazard factor is low atmospheric pressure, tested for vacuum.
- Process J: Hazard factor is concentrated impact, tested for impact.
Selecting Distribution Cycles
Distribution Cycle
A distribution cycle is a list of expected hazard (source) factors in the order in which they occur along the transportation route from production to consumption.
For sterile medical devices, the distribution cycle follows this sequence:
- Process A – Manual Handling
- Process C – Transport Stacking
- Process F – Unrestrained Vibration
- Process I – Low Pressure
- Process E – Transport Vibration
- Process J – Concentrated Impact
- Process A – Manual Handling
Sample Conditioning
Samples should be conditioned under standard atmospheric conditions, and any climate effects should be compensated for.
Unless other temperature and relative humidity conditions are considered more appropriate, transportation units should be conditioned at (23±1)°C and (50±2)% relative humidity according to ASTM D4332. A 72-hour conditioning period or sufficient time to allow all parts of the product and packaging to reach equilibrium is recommended. Tests should be conducted under conditioned atmospheric conditions, and if that is not possible, tests should occur soon after removing the samples from the conditioned environment. If needed, the transportation unit can be re-conditioned during the test procedure.
Process Descriptions
Process – Manual Handling
Manual handling tests are for individual containers, small packages, and any transport containers that can be manually handled, with a weight not exceeding 90 kg.
The test levels and methods are designed to evaluate the ability of the transportation unit to withstand the hazards (sources) generated by manual handling during the distribution cycle (such as loading, unloading, stacking, sorting, or palletizing). The main hazards are shocks caused by drops or tossing. The size, weight, and shape of the transport unit affect the degree of these hazards.

- For transport weight 0-9.1kg:
- Class I drop height: 610mm
- Class II drop height: 380mm
- Class III drop height: 230mm
- For transport weight 9.1-18.1kg:
- Class I drop height: 530mm
- Class II drop height: 330mm
- Class III drop height: 200mm
- For transport weight 18.1-27.2kg:
- Class I drop height: 460mm
- Class II drop height: 300mm
- Class III drop height: 180mm
- For transport weight 27.2-36.3kg:
- Class I drop height: 380mm
- Class II drop height: 250mm
- Class III drop height: 150mm
- For transport weight 36.3-45.4kg:
- Class I drop height: 300mm
- Class II drop height: 230mm
- Class III drop height: 130mm
- For transport weight 45.4-90.7kg:
- Class I drop height: 250mm
- Class II drop height: 180mm
- Class III drop height: 100mm
Process – Transport Stacking
This process tests the ability of the transportation unit to withstand compressive loads during transport stacking.
Process – Unrestrained Vibration
This process tests the ability of the transportation unit to withstand repeated vibrations during loose or unrestrained transport. The test considers vibration amplitude, direction, and duration.

Process – Low Pressure Test
This test simulates the pressure drop encountered when a packaged product is transported by certain modes of transport. It is suitable for products and packaging sensitive to low pressure environments, such as airtight sealed soft packaging, liquid containers, or breathable packaging.
Tests are carried out using an expected transport altitude, with a recommended pressure equivalent to 4267m altitude (59.5kPa) for 60 minutes. The test duration and pressure can be modified depending on the transport environment, product value, and acceptable damage levels.

Process – Transport Vibration
This test measures the ability of the transport unit to withstand dynamic compression forces caused by vertical vibrations during transportation.

Process – Concentrated Impact
This test evaluates the packaging’s ability to withstand concentrated external impacts during transport and handling. These impacts can result from goods colliding with each other during loading, unloading, or transfer processes. This test is particularly applicable to light single-wall corrugated packaging and plastic film-wrapped containers.
By following these processes, the ASTM D4169-2022 standard ensures that sterile medical device packaging is tested rigorously to maintain product integrity throughout its transportation lifecycle.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




