
Aesthetic Devices EU MDR Certification (EU 2017/745)
Preparing for EU MDR Standards:
The Medical Device Directive (MDD) was Europe’s previous regulatory and CE marking framework for medical devices, excluding most cosmetic and aesthetic products. However, the European Medical Device Regulation 2017/745 (MDR) covers many of these product types.
As a cosmetic or aesthetic product manufacturer, are you prepared to comply with the MDR now that it is fully in effect in the EU?
Cosmetic and Aesthetic Products Subject to MDR Compliance:
Appendix XVI of the MDR identifies the following cosmetic and aesthetic products that must comply with the new regulation:
1. Solid body contouring implants
2. Liposuction devices
3. Colored contact lenses
4. Skin fillers
5. Collagen implants
6. Laser/Intense Pulsed Light (IPL) hair removal devices
7. Skin resurfacing devices
8. Tattoo removal devices
MDR compliance means that cosmetic and aesthetic product manufacturers must obtain and maintain CE marking certification to sell their products in Europe.
What Does EU MDR Compliance Mean for Cosmetic and Aesthetic Product Manufacturers?
Despite the tight timeline, MDR compliance requires significant effort. Our testing consultants are ready to work with you to determine the most efficient and effective transition strategy for your company based on your product type and scope.
We can help you establish applicable MDR-related processes, such as:
1. Determining the risk classification of your device
2. Partnership with certification bodies for CE marking
3. Acting as the European Authorized Representative for companies without a European office
4. Implementing an ISO 13485:2016 Quality Management System
5. Establishing risk management processes
6. Identifying applicable common specifications for your device
7. Developing CE marking technical documentation
8. Post-market surveillance planning
9. Clinical Evaluation Reports (CER)
After evaluating your current operations, we can generate a comprehensive report on any potential MDR compliance gaps and recommend the most cost-effective methods to get your processes in place.
JJR Labs China is an IEC 17025 and GLP authorized laboratory. We have helped numerous medical device manufacturers obtain CE marking in Europe and possess the regulatory and quality expertise to help you quickly meet MDR requirements.
Email:hello@jjrlab.com
Write your message here and send it to us
 ASTM D4169 Drop Test
ASTM D4169 Drop Test
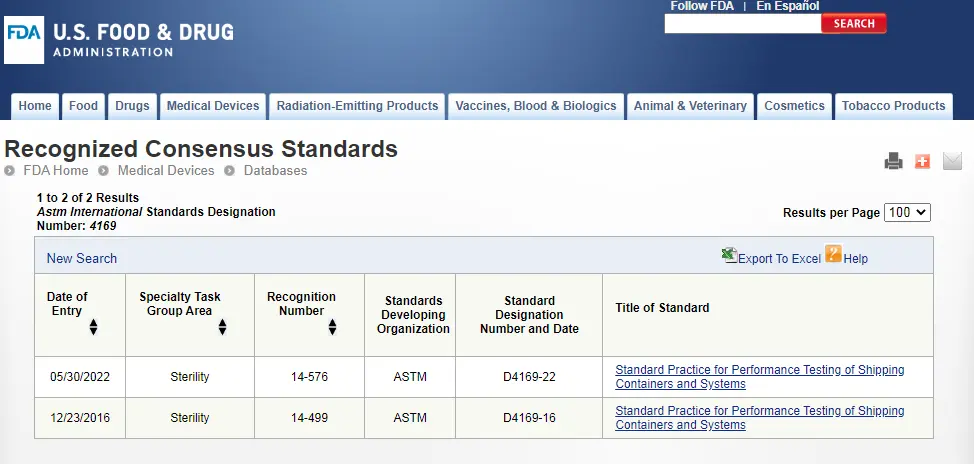 ASTM D4169 Packaging Simulation Transportation Tes
ASTM D4169 Packaging Simulation Transportation Tes
 What is ASTM D4169 Testing?
What is ASTM D4169 Testing?
 ASTM D4169-23 Test Standard Revision
ASTM D4169-23 Test Standard Revision
 Transport Simulation Testing for Medical Device Pa
Transport Simulation Testing for Medical Device Pa
 EU CE Certification Guidelines for Lighting Fixtur
EU CE Certification Guidelines for Lighting Fixtur
 Lithium Battery Export: CB Certification & IEC
Lithium Battery Export: CB Certification & IEC
 How to Apply for One FCC Certificate for Multiple
How to Apply for One FCC Certificate for Multiple
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




