
10993 Biocompatibility Testing
Medical Device biocompatibility testing - ISO 10993
The purpose of biocompatibility testing is to ensure that when materials come into contact with the human body, they do not release toxic substances that may cause local or systeMIC cytotoxicity, carcinogenicity, or reproductive toxicity. The material shoULd not cause inflammatory reactions, immune responses, toxic reactions, or thrombus formation after contact with the human body.
The JJR laboratory in China follows the widely recognized international standards used by health authorities, academia, and industry, namely the ISO 10993 series. In addition, the management of medical devices primarily refers to the U.S. FDA guidelines. Medical devices are classified into three risk categories based on their level of risk:
- Class I: Low risk
- Class II: Medium risk
- Class III: High risk
Only Class III devices and certain Class II devices require preclinical trials before market release. Relevant documentation and literature must be provided, such as preclinical trial results and non-clinical safety assessments (biocompatibility) from laboratories that adhere to Good Laboratory Practice (GLP), to demonstrate the safety and quality of the product.

Biocompatibility Test Introduction
When cells are exposed to toxic substances, they may undergo morphological changes such as vacuolation, detachment, lysis, and membrane integrity loss, possibly leading to cell death. The cytotoxicity test uses live cells to REDuce MTT (a yellow compound) in the mitochondria, generating a blue formazan crystal. Absorbance is measured to assess cell viability.
2. Skin Sensitisation Test
This test evaluates whether a medical device or material contains substances that can trigger contact allergic reactions or delayed-type allergic reactions (Type IV). It is one of the most sensitive tests for skin sensitization. After extraction, the substance is mixed with an adjuvant and injected into the skin of guinea pigs to amplify potential allergic reactions.
- Test Methods: Maximization Sensitization Test, Closed Patch Test
- Standards:
- iso 10993-10 Tests for skin sensitization
- OECD #406 Skin sensitization
3. Skin Irritation Test
This test evaluates whether a medical device or material contains ingredients that may cause skin irritation. After extraction, the substance is applied to the skin of rabbits via patches, and any potential irritant effects are observed.
- Standards:
- iso 10993-23 Tests for irritation
- OECD #404 Acute dermal irritation/corrosion
4. Intracutaneous Irritation Test
This test assesses whether a medical device or material contains substances that may cause intracutaneous irritation. After extraction, the substance is injected into the skin of rabbits, and any potential irritant effects are observed.
- Standards:
- ISO 10993-23 Tests for irritation
5. Eye Irritation Test
This test evaluates whether a medical device or material contains ingredients that may cause eye irritation. After extraction, the substance is instilled into the eyes of rabbits, and its effects on the cornea, conjunctiva, and iris are observed.
- Standards:
- ISO 10993-23 Tests for irritation
- OECD #405 Acute eye irritation/corrosion
6. Acute Systemic Toxicity Test
This test evaluates the potential harmful effects of a medical device or material through a single short-term exposure to the test substance or its extract in animals.
- Standards:
- ISO 10993-11 Tests for systemic toxicity
7. Pyrogen Test
This test determines if a medical device or material contains pyrogenic substances. The extract is injected into the ear vein of rabbits, and the resulting changes in body temperature are monitored.
- Standards:
- USP 151 Pyrogen Test
8. Endotoxin Test
Endotoxins are toxins produced by Gram-negative bacteria and are the most common pyrogens that cause fever reactions in humans. The endotoxin concentration can be measured using methods like turbidity or colorimetric assays. In turbidity tests, endotoxins cause clumping of reagents. In colorimetric assays, the reagent Boc-Leu-Gly-Arg-pNA reacts with endotoxins to produce a yellow color, and the concentration is determined using a standard curve.
9. Implantation Test
This test evaluates whether a medical device or material can cause local reactions when implanted in animals. The test substance is implanted into appropriate locations (subcutaneous, muscle, bone, etc.) in rabbits, and clinical observations, as well as histopathological analysis of tissue samples, are used to assess potential adverse reactions.
- Standards:
- iso 10993-6 Tests for local effects after implantation
10. Hemolysis Test
Hemolysis is the release of hemoglobin from damaged or partially damaged red blood cells, but with intact cell membranes. This test evaluates red blood cell damage through in vitro methods:
- Direct Contact Method: Detects hemolysis caused by physical factors (e.g., changes in osmotic pressure) or chemical interactions between substances and red blood cells.
- Extraction Method: Evaluates hemolysis caused by extracts from the test substance. Hemoglobin levels are measured using commercially available hemoglobin detection kits.
Email:hello@jjrlab.com
Write your message here and send it to us
 How Does a Product Get an Energy Star Label
How Does a Product Get an Energy Star Label
 Is ROHS part of UL the same
Is ROHS part of UL the same
 What is Protection Class EN 60529?
What is Protection Class EN 60529?
 IP69 Certified Protection
IP69 Certified Protection
 California Energy Commission Testing Lab
California Energy Commission Testing Lab
 What Does the Canadian IC Mark Mean?
What Does the Canadian IC Mark Mean?
 How Much is the Canada IC ID Certification cost?
How Much is the Canada IC ID Certification cost?
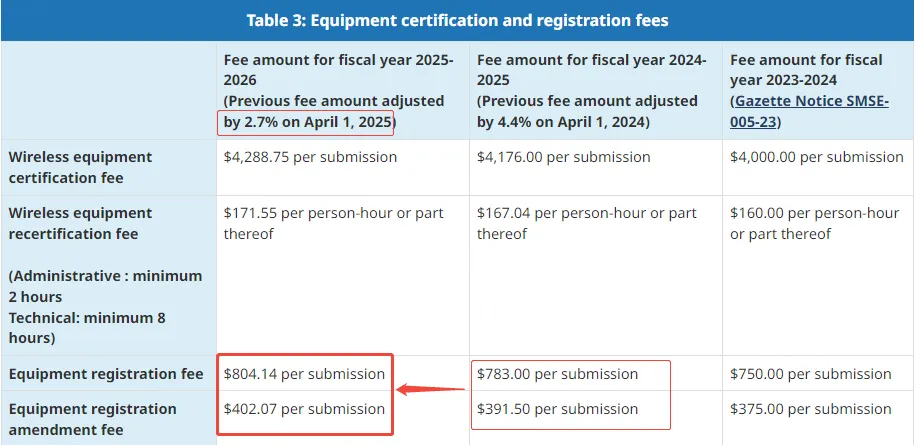 How Much is the Canada IC ID Certification Fee?
How Much is the Canada IC ID Certification Fee?
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




